You may recall from Gen chem (and no doubt your first week of o-chem as well), that orbitals on carbon come in two flavors: s and p.
s orbitals should be familiar as the spherical-shaped orbitals. The electrons of hydrogen, for instance, are in a 1s orbital.
p orbitals are shaped like figure-eights, or loops. The electrons in p orbitals are slightly farther away from the nucleus than those in s orbitals, so they are a little bit higher in energy. The p orbitals therefore fill with electrons only after the s orbitals are filled.
Hybridization is a concept that we address here.
What’s observed from analyzing the structure of molecules such as CH4 is that the shapes cannot result from the electrons being in s or p orbitals alone, but instead are a consequence of the electrons in s and p orbitals mixing to form hybrid orbitals. If you draw an analogy to how we could make a “hybrid” soft drink by mixing different proportions of Sprite and Pepsi, these new orbitals aren’t fully s or fully p, but are a combination of both. The “flavor” of each bond depends on the relative proportions of s orbital and p orbital content:
- sp3 = 25% s character, 75% p character
- sp2 = 33% s character, 66% p character
- sp = 50% s character, 50% p character.
It is these hybrid orbitals that form sigma bonds (σ bonds). Sigma bonds are created from the head-on overlap of orbitals. Those orbitals will be some combination of s orbitals and p orbitals.
[What’s happened to the missing p orbitals for sp2 and sp hybridized carbon? Well, these p orbitals aren’t involved in hybridization or in sigma bonding – instead, they maintain 100% p character, are available to form π bonds, which are created by the side-by-side overlap of orbitals. π bonding involves p orbitals exclusively. ]
Since carbon can exist in one of these three hybridization states, we can therefore have six varieties of carbon-carbon sigma bonds:
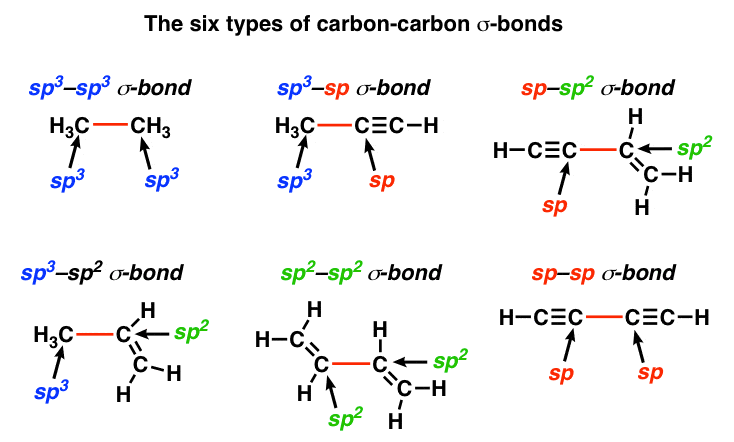
Now, recall that for any given quantum number, s orbitals are lower in energy than p orbitals. The electrons in s orbitals are held more closely to the nucleus than electrons in p orbitals. Take a look at the relative bond lengths and strengths for each of these six situations.
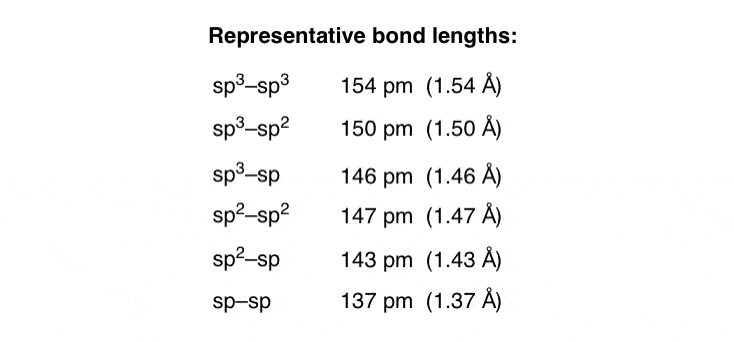
General principle – the more s character the bond has, the more tightly held the electrons will be.
Now, the number of π bonds that can form will be dependent on the number of unhybridized p orbitals available – 1 for sp2 hybridized carbons, 2 for sp hybridized carbons (the two π bonds will be at right angles to each other in the latter case).
In contrast to sigma orbitals, there is only one way to form a C-C π bond – from the overlap of two p orbitals.
Question: Relative to sigma bonds, do you think p-p bonds (π bonds) would be stronger or weaker?
1) What’s their % s-character?
2) Energy required to break C-C bond in ethane:
Energy required to break apart the C=C bond in ethene:
