Factors That Determine Whether A Species Is A Good Nucleophile
If you read the last post, you’ll recall that a nucleophile is a species that donates a pair of electrons to form a new covalent bond. Nucleophilicity is measured by comparing reaction rates; the faster the reaction, the better (or, “stronger”) the nucleophile.
Table of Contents
- Reminder: Nucleophilicity Is Measured By Reaction Rate
- The Role Of Charge: Nucleophilicity Increases As An Atom’s Electron Density Increases
- Electronegativity: Across The Periodic Table, Nucleophilicity Increases With Decreasing Electronegativity
- The Choice Of Solvent (Polar Protic vs. Polar Aprotic) Can Drastically Affect Nucleophilicity Trends
- Nucleophilicity Decreases With Increasing Steric Hindrance (“Bulkiness”)
- Notes
- (Advanced) References and Further Reading
1. Reminder: Nucleophilicity Is Measured By Reaction Rate
When discussing nucleophilicity we’re specifically talking about donating a pair of electrons to an atom other than hydrogen (usually carbon). When a species is donating a pair of electrons to a hydrogen (more specifically, a proton, H+) we call it a base.
This post attempts to address one of the most vexing question to students of organic chemistry. What are the factors that make a good nucleophile?
For our purposes, there are at least four key factors contributing to nucleophilicity.
- Charge
- Electronegativity
- Solvent
- Steric hindrance
The first two should hopefully be familiar from the discussion of what makes something a strong base. After all, basicity and nucleophilicity essentially describe the same phenomenon, except basicity concerns donation of lone pairs to hydrogen, and nucleophilicity concerns donations of lone pairs to all other atoms. It’s the third and fourth points where extra factors come into play.
2. The Role Of Charge: Nucleophilicity Increases As An Atom’s Electron Density Increases
Since a nucleophile is a species that is donating a pair of electrons, it’s reasonable to expect that its ability to donate electrons will increase as it becomes more electron rich, and decrease as it becomes more electron poor, right? So as electron density increases, so does nucleophilicity.
A handy rule to remember for this purpose is the following: the conjugate base is always a better nucleophile.
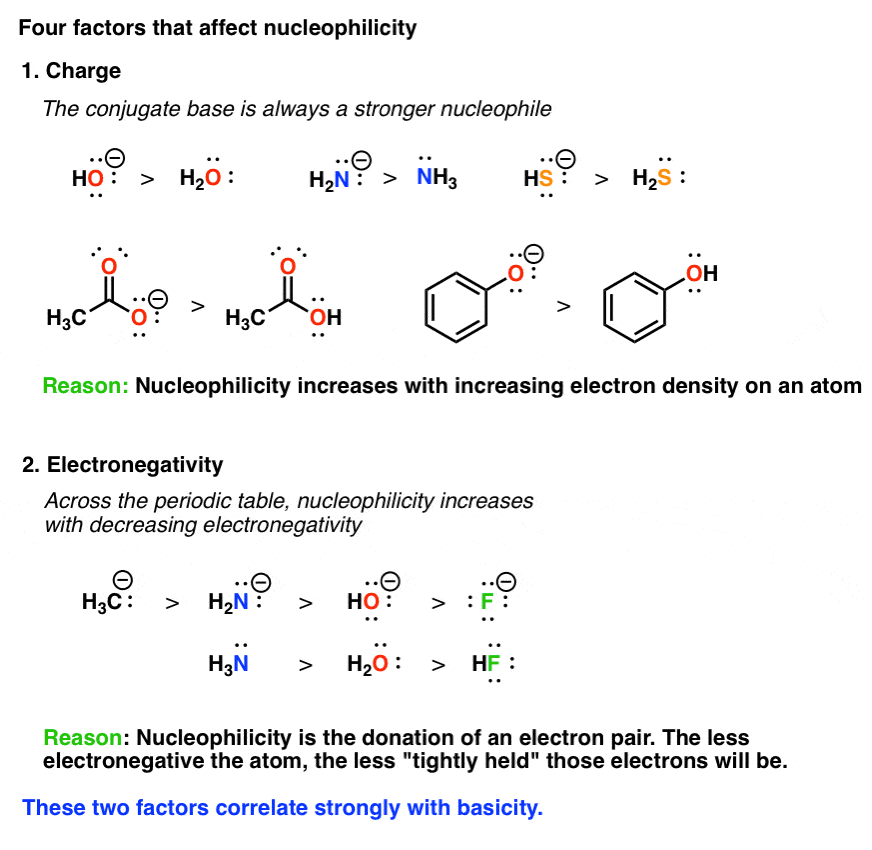
3. Electronegativity: Across The Periodic Table, Nucleophilicity Increases With Decreasing Electronegativity
Assuming an atom has a pair of electrons to donate, the ability of a species to donate that pair should be inversely proportional to how “tightly held” it is. The key factor for determining how “tightly held” an electron pair is bound is the familiar concept of electronegativity. Bottom line: as electronegativity increases, nucleophilicity decreases. Note: It’s important to restrict application of this trend to atoms in the same row of the periodic table; for instance, C N O F, or Si P S Cl. Going down the periodic table, another factor comes into play (next)
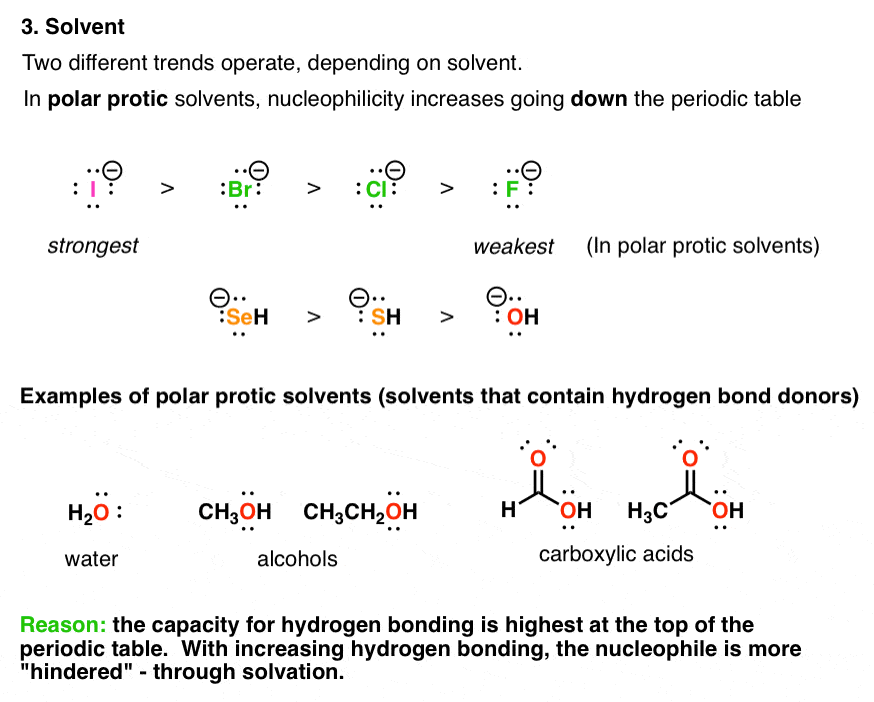
4. The Choice Of Solvent (Polar Protic vs. Polar Aprotic) Can Drastically Affect Nucleophilicity Trends
Nucleophilicity is not a property inherent to a given species; it can be affected by the medium it’s in (otherwise known as “the solvent”). [For an introduction to the different classes of solvents, see this post]
A polar protic solvent can participate in hydrogen bonding with a nucleophile, creating a “shell” of solvent molecules around it like mobs of screaming teenage fans swarming the Beatles in 1962.
In so doing, the nucleophile is considerably less reactive; everywhere it goes, its lone pairs of electrons are interacting with the electron-poor hydrogen atoms of the solvent.
The ability of nucleophiles to participate in hydrogen bonding decreases as we go down the periodic table. Hence fluoride is the strongest hydrogen bond acceptor, and iodide is the weakest. This means that in protic solvent, the lone pairs of iodide ion will be considerably more “free” than those of fluoride, resulting in higher rates (and greater nucleophilicity).
A polar aprotic solvent does not hydrogen bond to nucleophiles to a significant extent, meaning that the nucleophiles have greater freedom in solution. Under these conditions, nucleophilicity correlates well with basicity – and fluoride ion, being the most unstable of the halide ions, reacts fastest with electrophiles.
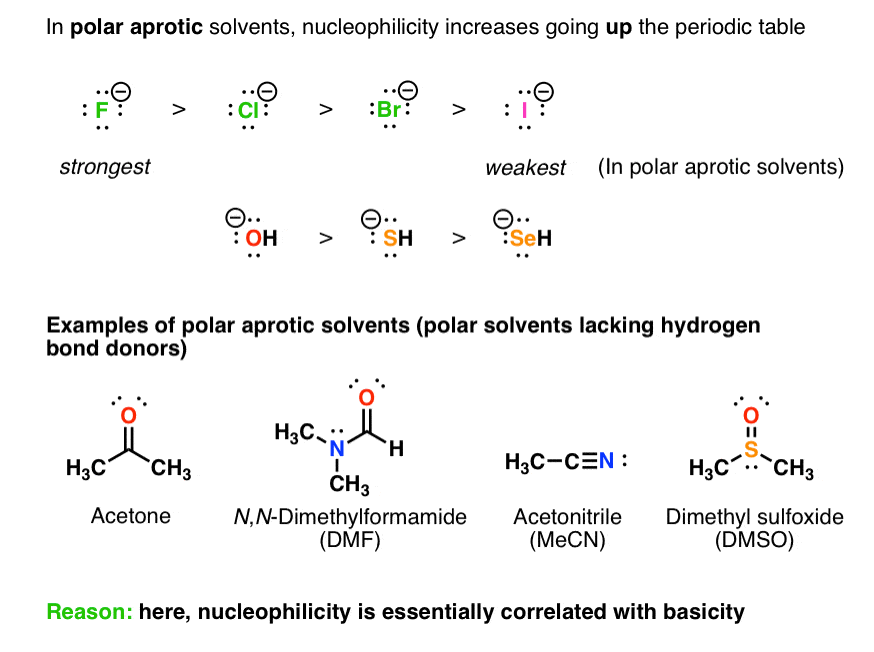
[Often asked: why don’t we care about “non polar solvents” here? Remember “like dissolves like”? If we want a reaction to take place, we need to use solvents that will actually dissolve our nucleophile. Many nucleophiles are charged species (“ions”) – they don’t dissolve in non-polar solvents.]
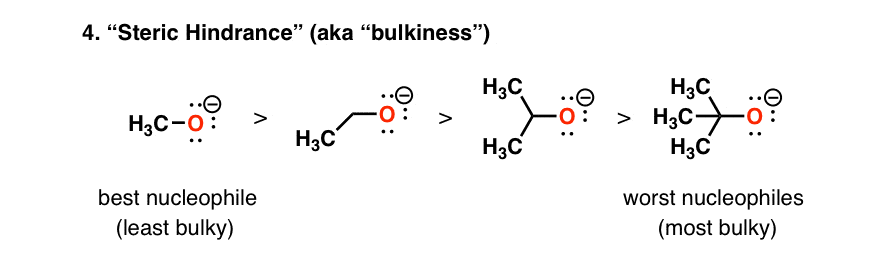
5. Nucleophilicity Decreases With Increasing Steric Hindrance (“Bulkiness”)
Since, when discussing nucleophilicity, we’re often discussing reactions at carbon, we have to take into account that orbitals at carbon that participate in reactions are generally less accessible than protons are. An effect called “steric hindrance” comes into play.
The bottom line here is that the bulkier a given nucleophile is, the slower the rate of its reactions [and therefore the lower its nucleophilicity].
So comparing several deprotonated alcohols, in the sequence methanol – ethanol – isopropanol – t-butanol, deprotonated methanol (“methoxide”) is the strongest nucleophile, and deprotonated t-butanol (“t-butoxide”) is the poorest (or “weakest”) nucleophile.