Good Leaving Groups Are Weak Bases
- A leaving group (a.k.a. “nucleofuge”) is the new Lewis base that is generated in various substitution and elimination reactions when a new bond is formed to carbon.
- Just as acid-base reactions favor reactions where a stronger acid plus a stronger base results in a weaker acid and a weaker base, substitution and elimination reactions tend to favor reactions where the leaving group is a weaker base than the nucleophile (or base, in the case of elimination reactions)
- Using pKa values it is possible to make good predictions as to whether various substitution or elimination reactions are favorable. Good leaving groups tend to be weak bases, i.e. the conjugate bases of strong acids (I–, Br–, Cl–, TsO–, H2O)
- Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a Lewis acid). For example, alcohols can be converted into alkyl chlorides through addition of HCl.
- Although the relative basicity of the nucleophile/leaving group is a good guide, a prominent exception is fluoride (F-) which is a weak base but tends not to act as a leaving group due to the extremely strong C-F bond (about 130 kcal/mol).
- When the basicity of the nucleophile and leaving group are comparable (i.e. within about 5-10 pKa units), an equilibrium may be established.
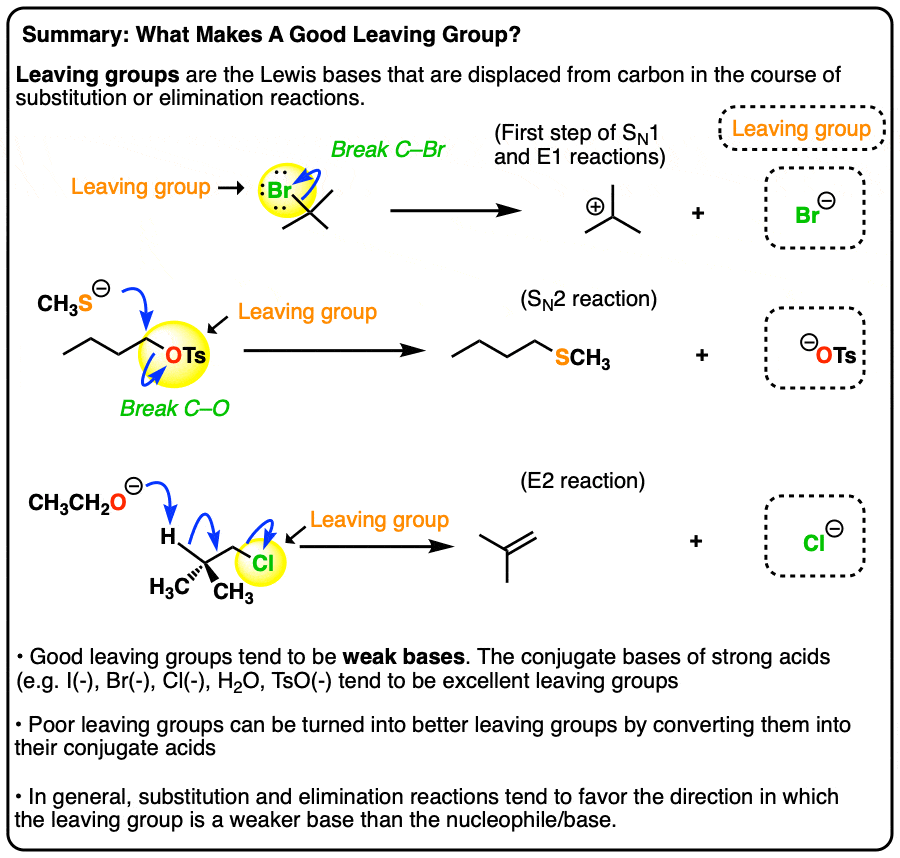
Table of Contents
- What Is A Leaving Group?
- The Principle of Acid-Base Mediocrity, Revisited
- Using a pKa Table To Evaluate Leaving Group Ability
- The Conjugate Acid Is A Better Leaving Group
- The Conjugate Base Is A Worse Leaving Group
- Exceptions – Fluorine
- The Best Leaving Group of All (N2)
- Summary
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. A Leaving Group Is Just A Nucleophile Going In Reverse
Many reactions that involve nucleophiles and bases result in the formation of a leaving group (also called a “nucleofuge“).
So what is a leaving group, anyway?
Well, a leaving group is just a nucleophile acting in reverse.
That is, whereas a nucleophile is the Lewis base that forms a new bond with a carbon, the leaving group is the Lewis base that results after breaking a bond with the carbon.
Here is an example of a reaction that produces a leaving group. This is a nucleophilic substitution reaction – specifically a nucleophilic substitution reaction at an alkyl carbon with with a bimolecular rate-determining step (SN2).
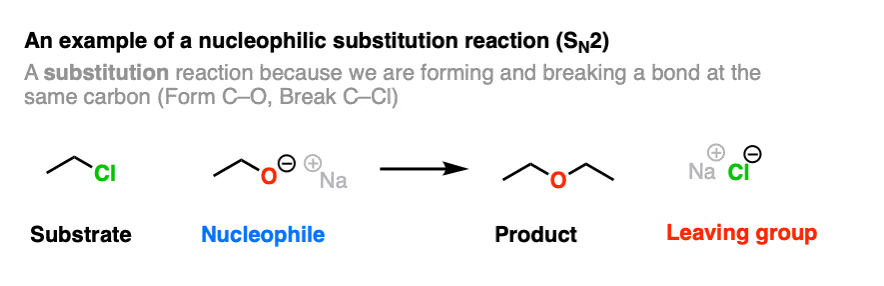
Note that this reaction has four components.
- A substrate (an alkyl halide in this case, which will be acting as an electrophile)
- A nucleophile (the CH3CH2O(-) ion)
- The product (the ether)
- The leaving group (the chloride ion, Cl(-) )
A C-O bond is formed, and a C-Cl bond is broken.
To see a few more examples of reactions that result in leaving groups we will cover below, hover here for a pop-up image or click on this link.
There is nothing stopping us from taking the reaction drawn above and flipping it around to give a reaction that goes in the opposite reaction. So let’s draw that out:
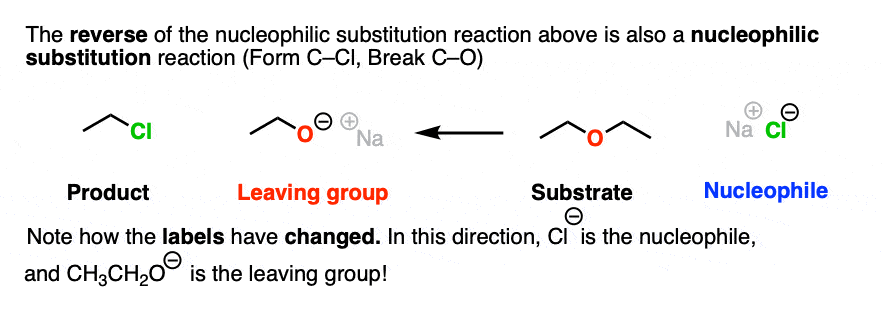
Note how in this drawing, the Cl(-) is the nucleophile and the RO(-) is the leaving group.
Note that I’m not saying that these two reactions are equally likely. Just because we can draw these two reactions does not mean that they are equally favored in a reaction vessel.
However, it is true that, as drawn, both of these reactions are nucleophilic substitution reactions, because we are forming and breaking a bond on the same carbon.
That being said: which reaction do you think is more favored?
In other words, if we ran the experiments, which reaction would you predict to proceed to completion?
 Click to Flip
Click to Flip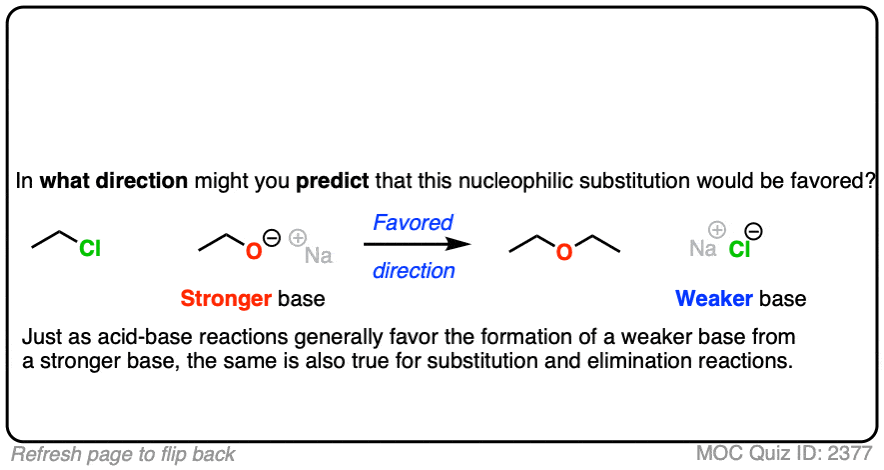
Congratulations if you said, the top one.
Why?
2. Are Substitution and Elimination Reactions Just Fancy Extensions of Acid Base Reactions?
Let’s change one little thing about that reaction above, and see if this looks a bit more familiar.
In the acid-base reaction between H-Cl and NaOCH2CH3 to give CH3CH2OH and NaCl, which direction would be more favored?
You might recall the Principle of Acid-Base Mediocrity: Stronger acid plus stronger base gives weaker acid and weaker base. (See post: How To Use a pKa Table).
So the favored direction of this acid-base reaction is formation of the weaker base (Cl -)
Just as acid-base reactions tend to be favored when we go from a stronger base to a weaker base, substitution and elimination reactions also tend to be favored when the leaving group is a weaker base than the nucleophile (or base).
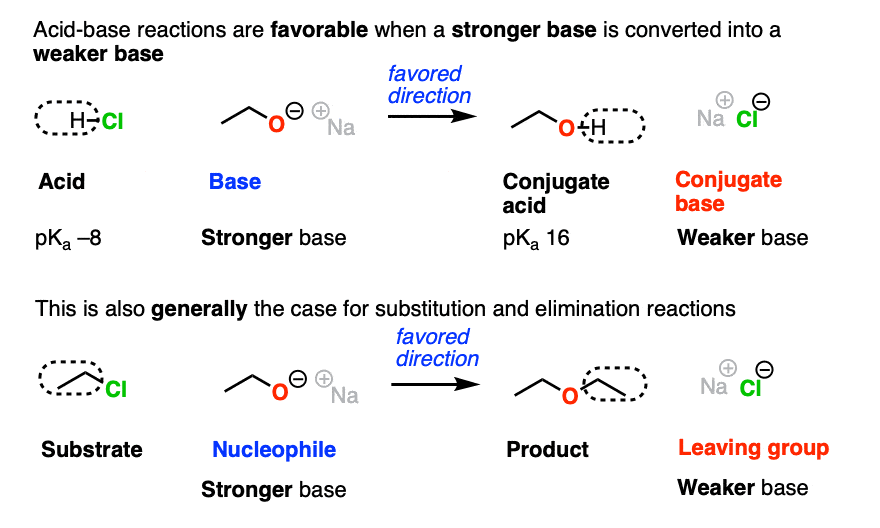
In fact, the four components of an acid base reaction map pretty well onto the four components of the nucleophilic substitution reactions, above.
- Acid → substrate
- Base → nucleophile
- Conjugate acid → product
- Conjugate base → leaving group
The substitution above also favors formation of the weaker base (Cl-) from the stronger base (CH3CH2O-)
For that matter, so does this elimination reaction
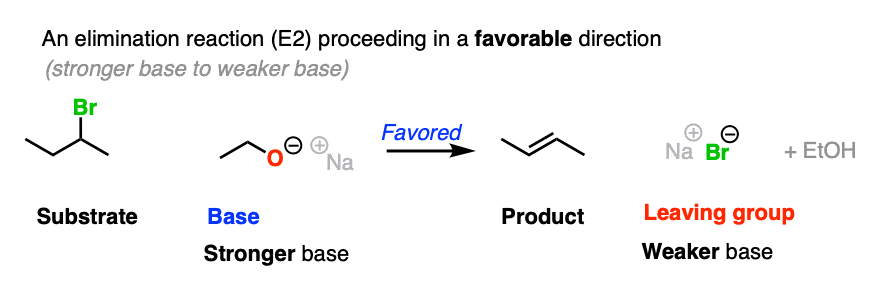
If the leaving group and base are flipped, the reaction is much less favored. To see this “flipped” reaction, hover here for a pop-up image or click on this link.
This is also the case with the loss of leaving group in this SN1 reaction:
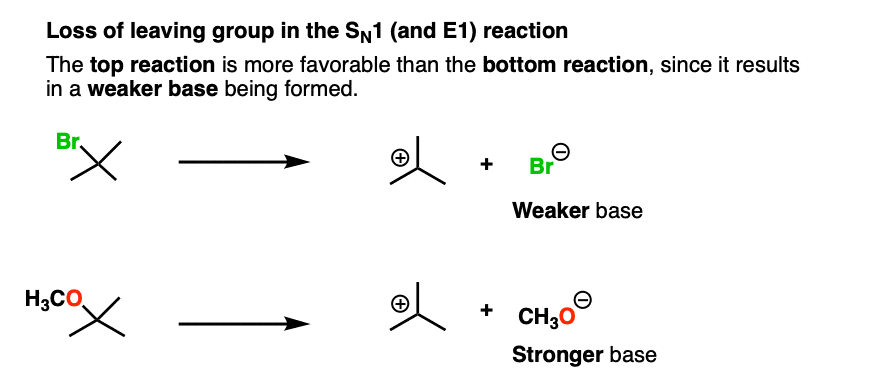
There are many more examples, like these nucleophilic acyl substitution reactions that you will see in Org 2. To see an example, hover here for a pop-up image or click on this link.
Generally speaking, the weaker the base, the better the leaving group.
We can reverse this and also say that the stronger the base, the worse the leaving group.
3. Using a pKa Table To Determine Leaving Group Ability
If base strength determines leaving group ability, then a pKa table should be a pretty good guide to determining leaving group ability.
In a previous post (See: How To Use a pKa Table) we went through this in considerable detail.
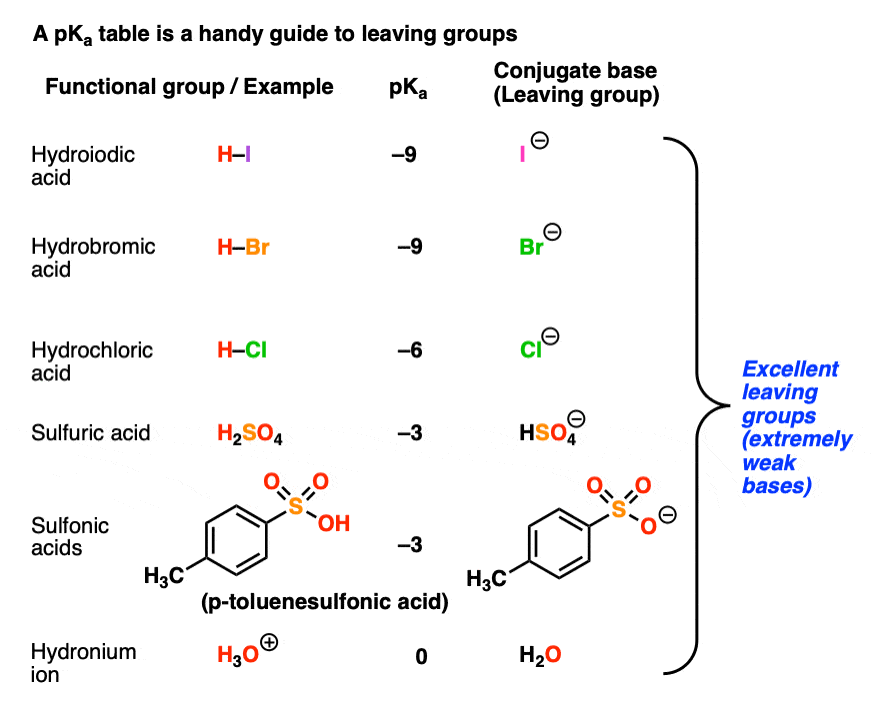
Recall that the stronger the acid, the weaker the conjugate base.That means that the top portion of a pKa table should be an excellent guide to the best leaving groups. The conjugate bases of strong acids, such as I(-), Br(-), Cl(-), HOSO3(-), TsO(-), H2O, and others are weak bases and great leaving groups.
Here is another example of a favorable reaction, where the nucleophile is more basic than the leaving group:
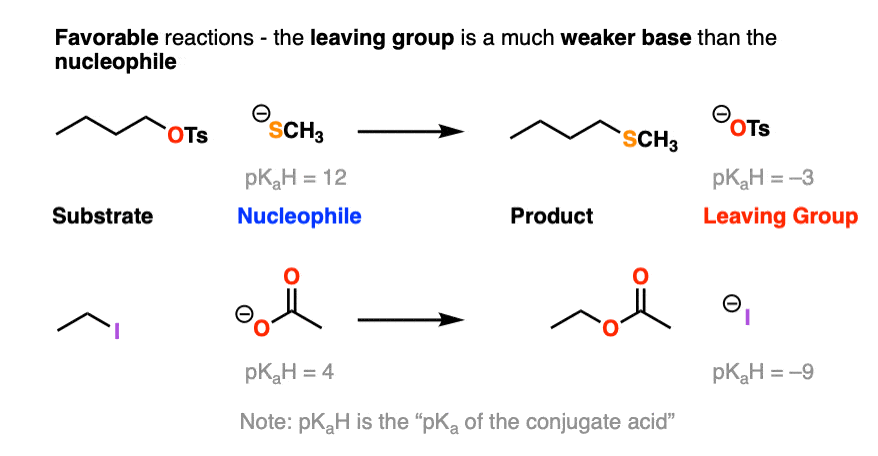
Note that pKaH is shorthand for “the pKa of the conjugate acid”.
On the other hand, the bottom part of a pKa table should be a guide to the worst leaving groups – the conjugate bases of weak acids, such as H(-) and H3C(-) are extremely strong bases.
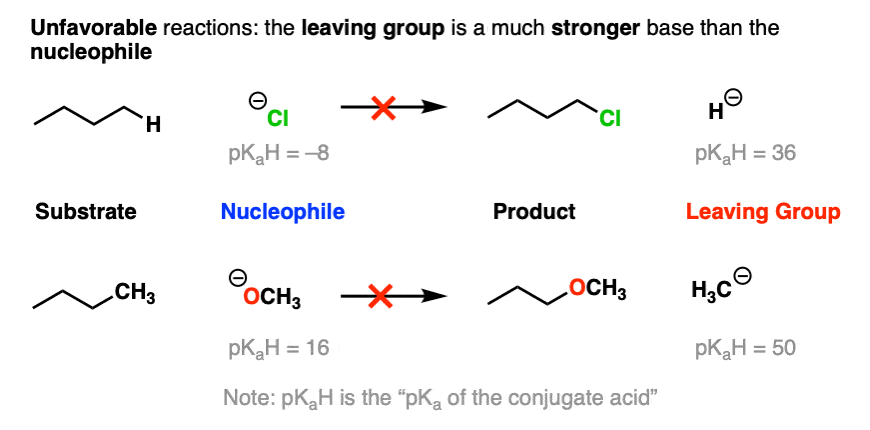
This is why we never see alkyl groups or hydride ions as leaving groups.
I should stress that comparing the basicity of the nucleophile and leaving group is a good place to start, but is not the only variable. The relative strength of the bonds being broken and formed is also a factor, which comes up most prominently with fluorine (see Exceptions, below).
When the nucleophile and leaving group are of comparable basicity (i.e. within about 5-10 pKa units), substitution reactions are potentially reversible, and the equilibrium can be driven to one side or the other depending on what conditions we choose. [Note 1]
Also, it’s important to remember that acidity and basicity (i.e. pKa) are measured by the position of an equilibrium (think “differences in energy”) whereas nucleophilicity is measured by reaction rates since many reactions of nucleophiles are irreversible. So although the correlation is very good, it isn’t perfect.
Also, be on the lookout for acid-base reactions. A good rule of thumb is that acid-base reactions are fast, relative to substitution and elimination reactions (see post: Acid-Base Reactions are Fast)
4. The Conjugate Acid Is A Better Leaving Group
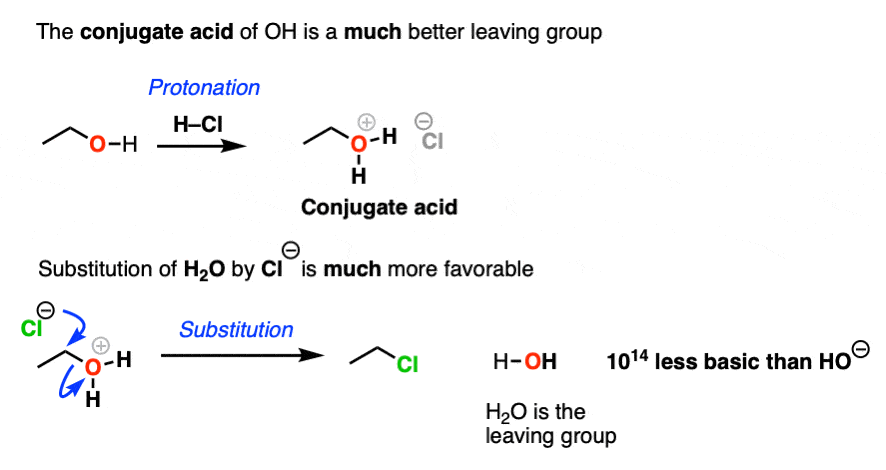
Hydroxide ions are poor leaving groups because they are strong bases.
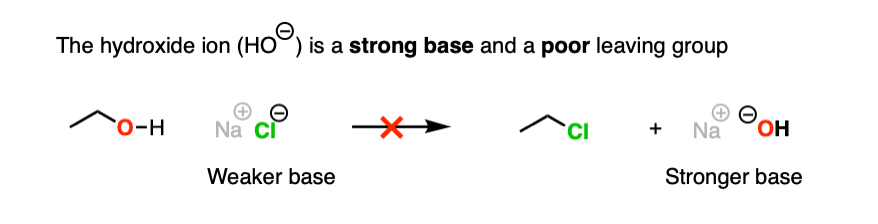
We might ask ourselves: is there some way to modify the OH so that when the C-O bond breaks, the leaving group is a weaker base? Then it would be more favorable.
Yes, there is a simple and effective way to make OH a better leaving group.
If we just add strong acid, then we make the conjugate acid of R-OH, which is R-OH2(+).
Now, if Cl(-) attacks, our leaving group will be the weak base H2O. This is a factor of 1014 less basic than HO(-).
So in the presence of acid, this reaction becomes much more favorable. The conjugate acid is a better leaving group! (This is one of the major applications of acid catalysis in organic chemistry.)
In the chapter on alcohols, we’ll see that many substitution and elimination reactions of alcohols require acid in order for the OH group to leave.
Lewis acids can be used for this purpose as well. [Note 2] We will see many examples of this in the chapter on electrophilic aromatic substitution (e.g. in the Friedel-Crafts reaction where Lewis acids like AlCl3 are used to make Cl into better leaving groups (See post: The Friedel Crafts Alkylation and Acylation Reactions).
Other ways to modify -OH to make it into a better leaving group includes transforming them into sulfonates, which are much weaker bases than HO(-). [See post: Tosylates and Mesylates] [Note 3]
5. The Conjugate Base Is A Worse Leaving Group
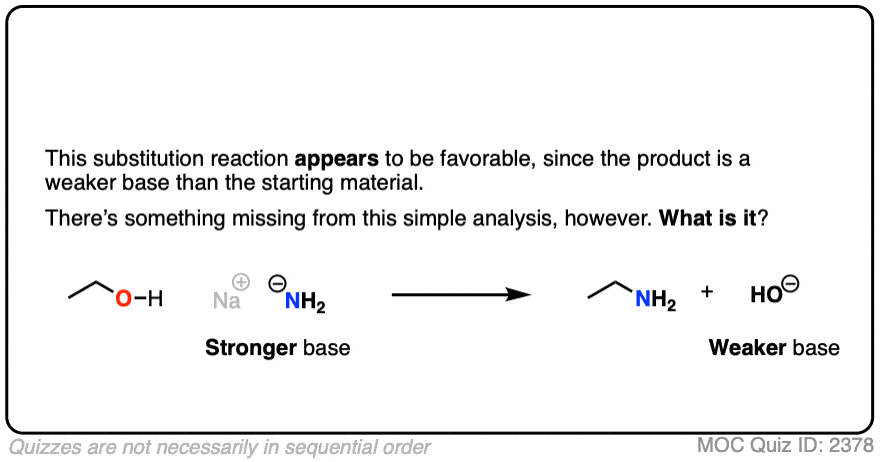
It’s true that the conjugate acid is a better leaving group. It’s also worth noting that the conjugate base is a worse leaving group.
Also, acid-base reactions are faster, generally speaking, than substitution and elimination reactions.
If a strong base is added to a species with an acidic proton, the acid-base reaction will occur first. This will give the conjugate base, which will possess a much worse leaving group than the neutral species.
Here’s an example. You might think the leaving group here is HO(-) . It’s not. The leaving group would have to be O(2-)
 Click to Flip
Click to Flip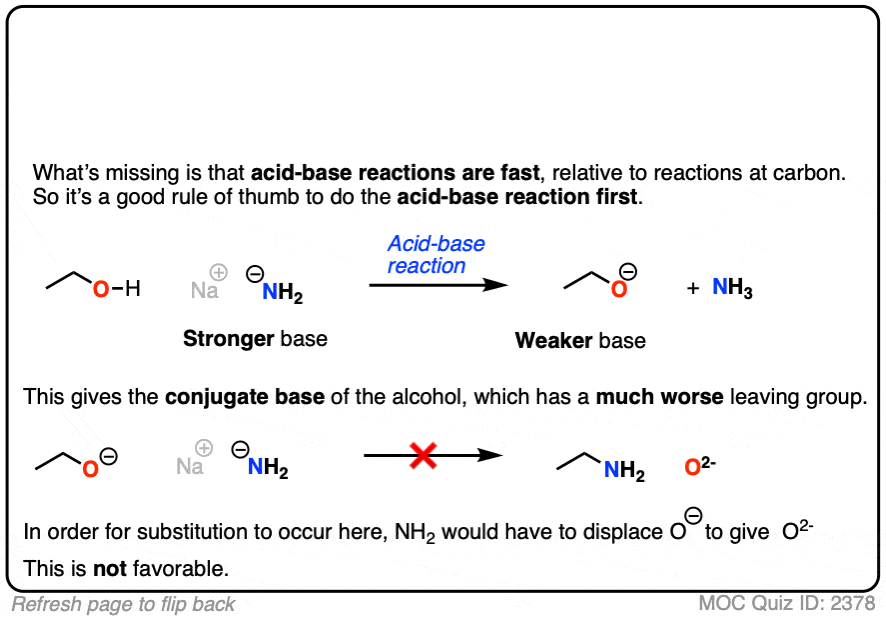
That’s a much worse leaving group.
Along the same lines, don’t forget that carboxylic acids…. are acids!
6. Exceptions
Is that all there is to it? Just apply the Principle of Acid-Base Mediocrity and we’re done with it?
Not quite. It wouldn’t be organic chemistry if we didn’t mention some exceptions.
One prominent exception is fluorine. Fluoride ion (F-) is a weak base (the pKa of HF is 3.8) , but generally a poor leaving group in substitution reactions. [Note 4]
One key reason is the strength of the C-F bond (about 130 kcal/mol).
Up to this point, we’ve only really considered the stability of the leaving group relative to the nucleophile. But the stability of the product is important as well.
Since the C-F bond is so strong, the reverse reaction (breaking the C-F bond, giving F(-) as a leaving group) would have an extremely high activation energy, making it very unfavorable.
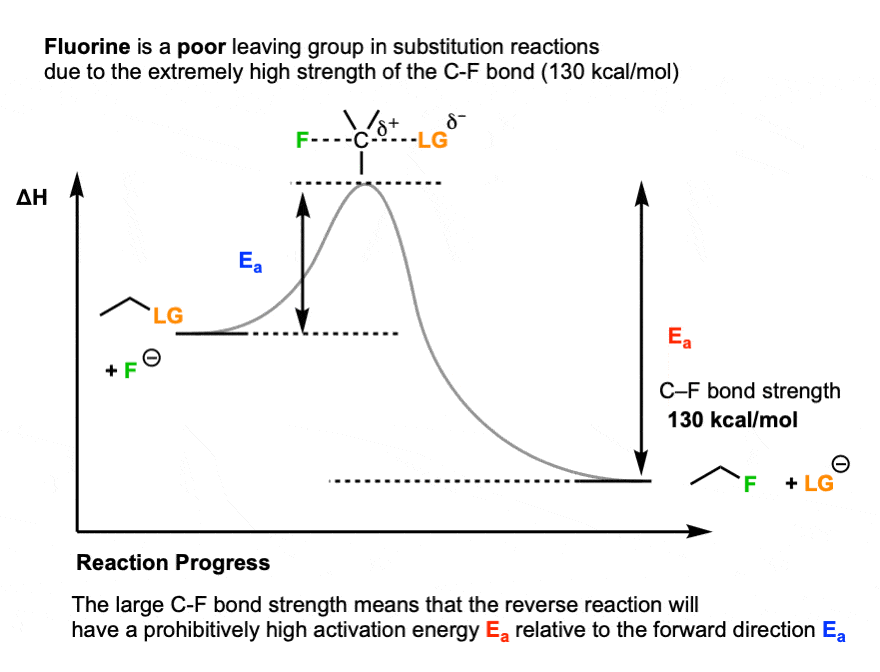
So the key variable is not just the relative stability of the nucleophile versus the base, but also the strength of the bond that is forming and breaking.
Another example of a reaction where fluorine is a leaving group is nucleophilic aromatic substitution, but the breaking of C-F is not the rate-determining step in this case (See Post: Nucleophilic Aromatic Substitution)
7. The Best Leaving Group Of All
Probably the best leaving group of all is dinitrogen (N2). Not only is nitrogen exceptionally stable – after all, it’s 79% of our atmosphere and is essentially inert – it is a gas, which means that any organic reaction where N2 is displaced will result in it bubbling out of solution, rendering the reaction essentially irreversible (sometimes explosively so!).
Amines (R-NH2) can be converted into diazonium salts R-N≡N(+) with nitrous acid (HONO) through a process called “diazotization”.
However dinitrogen is such a good leaving group that sp3-hybridized carbon atoms bonded to N2 are exceptionally unstable and tend not to stick around long enough to participate in reactions with nucleophiles; once formed, the N2 just leaves, resulting in carbocations (and not uncommonly, shattered glassware) in its wake.
Diazonium salts of sp2-hybridized carbons are considerably more stable and used in organic reactions; for more information, see this post (See post: Reactions of Diazonium Salts)
8. Summary
Let’s review.
- Leaving groups are nucleophiles acting in reverse – i.e. they are Lewis bases formed from the breakage of a bond to carbon.
- Just as acid-base reactions are favored in the direction which results in a weaker base being formed from a stronger base, reactions involving leaving groups also tend to be favored in the direction which gives the weaker bas.
- The conjugate bases of strong acids (e.g. I-, Br-, Cl-, TsO-, H2O) are good leaving groups.
- The conjugate base of weak acids (H-, H3C(-), alkyl anions) are poor leaving groups.
- The conjugate acid is a better leaving group. One common example is with alcohols, which can undergo substitution and elimination reactions after the HO is protonated.
- The conjugate base is a worse leaving group. A good rule of thumb is to do acid-base reactions first.
- Fluoride ion (F-) is a weak base, but a poor leaving group due to the strength of the C-F bond.
If you’re not sure where a reaction is going to happen on a molecule, look for a good leaving group. That’s usually where the action is!
