What Bonds Form, What Bonds Break?
About a month or so ago I had a big revelation as an instructor. Something that I’d been taking for granted, that was right under my nose.
I was spending so much time focusing on the why and how of chemical reactions, that I had neglected to make sure that students understood the what – in detail.
I was assuming that the vast majority of students could look at a reaction and quickly see which bonds were being formed and which were broken.
I was assuming this because I’d always ask “do you have any questions about this?” and they’d say “no”. So I assumed that they had possession of this most basic and important skill.
No no no no no.
Because when I thought to ask, “what specific bonds are formed, and what specific bonds are broken”, the variance in how quickly (and correctly) students could answer would be HUGE.
Take this reaction:
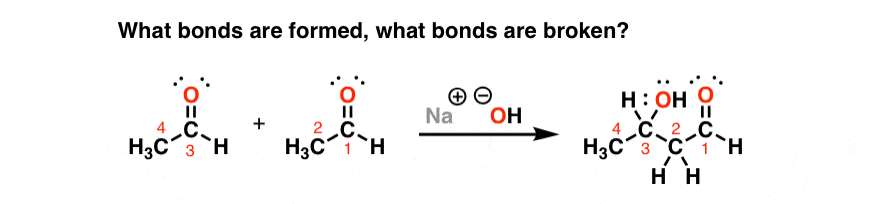
Now ask the question. What’s different? What bonds formed, and what bonds broke?
Someone professionally trained in organic chemistry could answer this question as quickly as they can speak.
An A-caliber student would take just a little bit longer.
But for someone struggling with organic chemistry, on the threshold between success and failure, answering this question could take minutes. I’ve seen it happen.
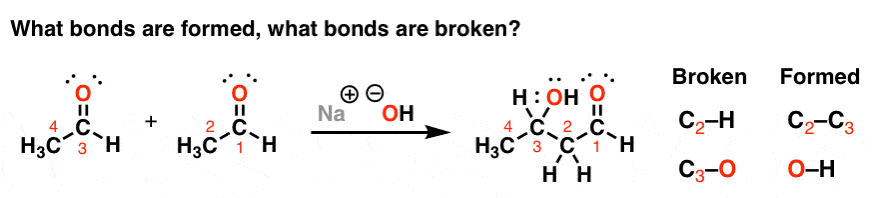
I think because I’ve spent the better part of the past 10 years talking with other Ph.D. caliber organic chemists, I couldn’t imagine someone not being able to see this. It’s like trying to imagine not being able to read English. But now things are much clearer.
I think that asking “what bonds were formed, and what bonds were broken” is the most important question you can ask about a chemical reaction. After all, knowing the answer to “what happens” is a prerequisite to being able to answer “how it happens”, or “why it happens”.
And it’s a completely learnable skill. It’s a skill that someone could employ coming right out of gen chem. In fact, gen chem students do it all the time. Remember Hess’ law? Figuring out the enthalpy of a reaction by seeing which bonds break and form?
The other thing is, it’s a question you can answer without knowing how the reaction works. The only thing you need is to be able to read chemical structures – to be able to figure out which atoms are bound to what. (That’s not trivial – a fair amount of effort in the first week or two of Org 1 is devoted to just that topic. In this case you’ll need to know about all the atoms that are hidden in these line drawings.)
It’s also a question you can ask about every chemical reaction you will ever encounter.
From over 1000 hours of 1-on-1 tutoring, I don’t know that I’ve found anything that has a bigger educational impact than making sure students get this question right. It’s even more important than arrow pushing, in my opinion.
