Energy Diagram Of The Cyclohexane Chair Flip
In the last post, we showed a video of a cyclohexane ring flip – turning a cyclohexane chair conformation into a boat and then into the opposite chair.
The key observation we made here was that a chair flip converts all axial groups into equatorial groups and all equatorial groups into axial groups. However all “up” groups remain up and all “down” groups remain down.
Table of Contents
- How Much Does A Cyclohexane Chair Flip “Cost” ? About 10 kcal/mol
- The Cyclohexane Chair Flip Energy Diagram
- The Energy Barrier For A Cyclohexane Chair Flip Is Small Enough To Allow The Two Conformations To Interconvert At Room Temperature
- Summary: The Cyclohexane Chair Flip Energy Diagram
- Notes
- (Advanced) References and Further Reading
1. How Much Does A Cyclohexane Chair Flip “Cost” ? About 10 kcal/mol
Now that we know what “looks” like to do a chair flip, let’s ask a different question: how much does it “cost”? When we say “cost”, of course, we’re talking about energy. Many organic chemists like to use kcal/mol [to convert to kJ/mol, multiply by 4.184] (See post: Why do Organic Chemists Use Kilocalories)
As you might have noticed while watching the video, converting one cyclohexane into the opposite chair conformation isn’t a matter of doing a simple bond rotation, like it is for, say, “eclipsed” butane into “staggered’ butane. There’s a lot more going on – each C-C bond undergoes rotation of some form.
Let’s walk through it in more detail.
Chair (ground state = 0 kcal/mol) –> Half Chair (+10 kcal/mol above ground state)
First, we take one end of the cyclohexane chair and push it into the “plane” created by the four carbons, making a “half chair”. This first step is actually the most unfavorable – because of a combination of ring and angle strain, the half-chair lies 10 kcal/mol in energy above the chair conformation.
Half-Chair (+10 kcal/mol) –> Twist-Boat (+5.5 kcal/mol)
The next step is to continue pushing that “end” of the half chair up until it is roughly on the same level as the other “end”. This makes a “twist boat”, which is a local energy minimum – we no longer have angle strain (all bonds are again 109°) but there is some torsional strain owing to the fact that there are two pairs of eclipsed C-C bonds. There is also a “flagpole” interaction between the hydrogens on the “prows” but in the twist-boat, they are slightly offset with respect to each other. The twist-boat is 5.5 kcal/mol in energy above the cyclohexane chair.
Twist-Boat (+5.5 kcal/mol) –> Boat (+6.5 kcal/mol) –> Twist Boat (+5.5 kcal/mol)
Where to go from here? Well, the “twist” momentarily passes through a full “boat” conformation (6.5 kcal/mol) on its way to a different “twist”, which is a bit awkward – in the full boat the two “flagpole” hydrogens are held in very close proximity to each other (within each other’s Van Der Waals radius). Think of two friends with long Cyrano de Bergerac noses kissing each other on alternate cheeks – there’s an awkward moment when they briefly bang noses in the middle : – )
Twist-Boat (+5.5 kcal/mol) –> Half Chair (+10 kcal/mol) –> Chair (0 kcal/mol)
From the new twist, we’re merely going backwards to get to the alternate chair – down goes one “prow” to give (momentarily) a half-chair, en route to the flipped chair.
2. The Cyclohexane Chair Flip Energy Diagram
If we draw an energy diagram, the whole process looks like this. Again, note that the chair on the left has the red hydrogens axial, and in the chair on the right, the red hydrogens are now equatorial.
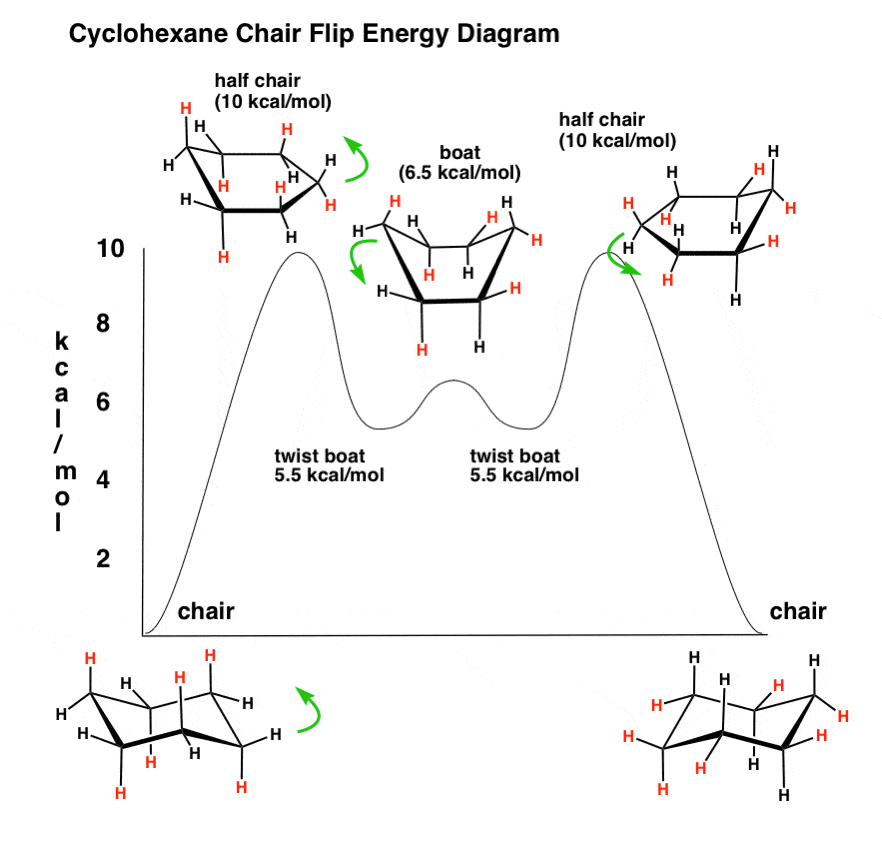
So what? you might ask. We’ve turned one chair into another. Who cares?
[And you might not care. That’s fine. The following discussion is not crucial for us going forward, but is helpful to understand a key consequence of this energy diagram…. ]
3. The Energy Barrier For A Cyclohexane Chair Flip Is Small Enough To Allow The Two Conformations To Interconvert At Room Temperature
For cyclohexane, I cede your point of “who cares”, because for all purposes the two chair forms are identical.
However, things start getting interesting once we start putting any type of substituent on our cyclohexane.
For example, let’s take 1-methylcyclohexane. Let’s say we start with the chair on the left (methyl is axial) and a chair flip converts it into the chair on the right (methyl is equatorial).
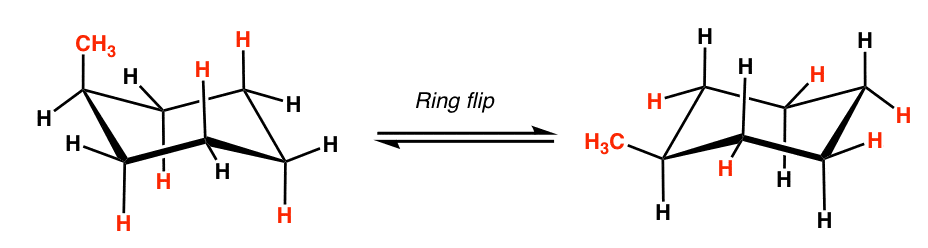
First of all, note that these are NOT mirror images of each other – they are different conformations.
Being quite rigid molecules, you would expect “axial” 1-methylcyclohexane to have slightly different properties than “equatorial” 1-methylcyclohexane.
If you could isolate the “axial” conformer, for instance, you’d expect it to have a slightly different melting point and boiling point than the “equatorial” conformer, since the molecules will stack differently with each other.
Furthermore, with other cyclohexanes, the axial and equatorial conformers even have different reactivity (more to come on this in a future chapter).
So how do we handle these differences? Well, we pretty much ignore it. The energy barrier of 10 kcal/mol for this interconversion is large, but not large enough that it prevents these two conformations from interconverting at room temperature (300 Kelvin). In fact these two conformations do interconvert, many times per second.
For our purposes, the bottom line is that you can consider the two chair conformations of 1-methylcyclohexane to be in equilibrium with each other, and furthermore, the properties of the bulk will be a weighted average of the two conformations. [Note 2]
[At low temperatures it’s a different story and the two conformations are “trapped” – we’ll cover that in Note 1 below. ]
4. Summary: The Cyclohexane Chair Flip Energy Diagram
In this article the cyclohexane chair flip energy diagram was very simple because the two chair forms are exactly equal in energy. In the next post, we consider the possibility that for substituted cyclohexanes (such as 1-methylcyclohexane) the two chair forms are NOT equal in energy.
First, why might that be? And second, how might that affect the population of the two conformations?
Answers in the next post…
Next Post: Substituted Cyclohexanes – Axial And Equatorial
Notes
Note 1. Substituted Cyclohexanes And The NMR Timescale
Imagine we have a magical device that can take “snapshots” of molecules, so that we can tell, at any given time, what the structure of a molecule is. Press a button, and presto ! you get pictures of all the molecules in solution.
What would a “snapshot” of a solution of “1-methylcyclohexane” look like? Assume that 1) the molecules will spend >99% of their time in “chair” conformations, and 2) assume (for now) that the two chair forms are equal in energy.
Based on this, we’d expect to see that 50% of the “snapshots” show “axial” 1-methylcyclohexane, and 50% show “equatorial” 1-methylcyclohexane.
[We actually do have a device which does this, and it’s not magic – it’s called an NMR spectrometer – more on that in a later series].
So what do we actually see?
Here’s the cool part.
At very low temperatures (–78°C, which is the temperature of the cheap dry ice/acetone cold bath) our “magic device” does indeed show that there is a mixture of equatorial and axial 1-methylcyclohexane in solution, just like we might expect.
However, when we let it warm up to room temperature, something interesting happens. The “snapshots” of the “axial” and “equatorial” 1-methylcyclohexane start blurring together, until we see a single signal that is an average of those two snapshots.
So what could explain this? Why might we see two “snapshots” at low temperature, but a single, “blended” snapshot at high temperature?
Does it remind you of something? Maybe of the difference between taking pictures of a ceiling fan at rest (where you can see the individual blades) and taking pictures at high speed (where you just see a blur). Or spokes on a bicycle? Or a colour wheel?
We see a “blur” on our magic snapshot machine (i.e. an NMR spectrometer) because, like a camera with a long shutter speed, we see an average of the different states over time.
A similar type of thing is happening here. At low temperatures, we have separate populations of “axial” and “equatorial” 1-methylcyclohexane, which do not have sufficent energy to ascend the 10 kcal/mol barrier (through the “half-chair”) that would allow for their interconversion.
At higher temperature, there is sufficient energy for each molecule to ascend the barrier to half-chair formation, and therefore the interconversion can occur.
Note 2 – Chiral conformations . Soon enough you will cover chirality. With some substituted cyclohexanes (e.g. cis-1,2-dimethylcyclohexane) you may note that each chair conformation is chiral, and the two chair conformations are enantiomers of each other. However, the molecule is considered to be “achiral” overall since the two chiral enantiomers are in equilibrium with each other and the optical activity of these two conformations cancels out. That’s what I meant by “the properties of the molecule as a whole will be the weighted average of the conformations. ”
