Rearrangement Reactions: Substitution Reactions With Hydride Shifts
In this post we cover several examples of reactions where carbocations form… but then a funny thing happens. An adjacent bonding pair of electrons (i.e. a C-H bond) interacts with the empty p-orbital, and before you know it, the C-H bond has moved and a new, more stable carbocation has formed! The carbocation is then attacked by the nucleophile, giving a substitution reaction (SN1) with rearrangement!
Table of Contents
- Spotting A “Substitution With Rearrangement”: An Extra Set Of C-H Bonds Forms And Breaks
- Carbocation Stability: Tertiary > Secondary >> Primary
- If A Less Stable Carbocation Can Be Transformed Into A More Stable Carbocation Through The Migration Of A C-H Bond, Then A Rearrangement Is Possible
- Examples Of “Allowed” Carbocation Rearrangement Reactions That Occur Through Hydride Shifts
- The SN1 Reaction With Hydride Shift: Arrow Pushing Mechanism
1. Spotting A “Substitution With Rearrangement”: An Extra Set Of C-H Bonds Forms And Breaks
For nucleophilic substitution, the pattern of bonds that form and break is pretty straightforward. You break C-(leaving group) and you form C-(nucleophile). A straight swap. But every once in awhile you might see a “weird” substitution reaction. If you look closely at the pattern of bonds formed and bonds broken in the second reaction below, there’s an extra set!
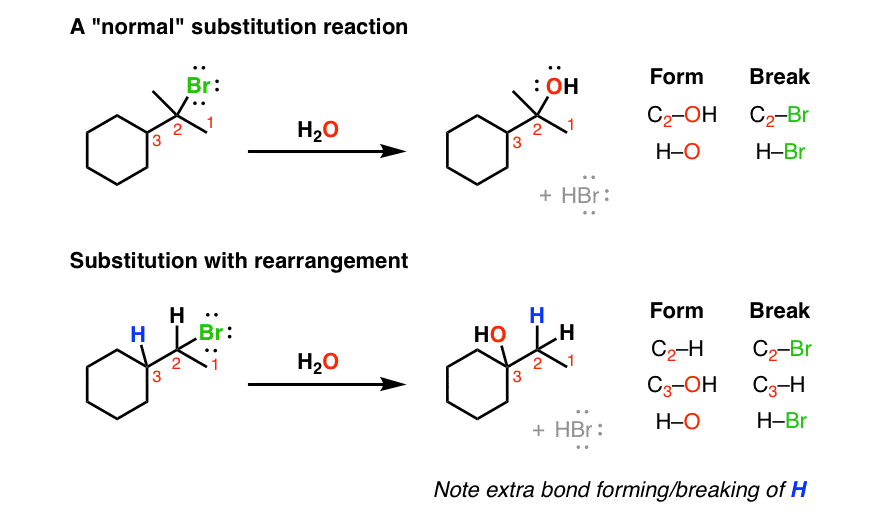
In other words it’s a substitution reaction where the hydrogen has moved. We call these movements “rearrangements”, for reasons that will become clear shortly.
The big question is, what’s going on? How did this happen?
2. Carbocation Stability: Tertiary > Secondary >> Primary
As it turns out, reactions that go through carbocations can sometimes undergo rearrangements. And looking back at substitution reactions, recall that theSN1 reaction goes through a carbocation intermediate. (See post: The SN1 Mechanism)
In this post we’ll go through when you’ll expect to see a rearrangement reaction.
Let’s think back to carbocations. They’re carbon atoms with six electrons bearing a positive charge. In other words, they’re electron deficient – 2 electrons short of a full octet.
So it would make sense that carbocations become more stable as you increase the number of electron donating groups attached to them. Alkyl groups are a perfect example. That’s why carbocation stability increases as you go from primary to secondary to tertiary. (See post: Carbocation Stability)
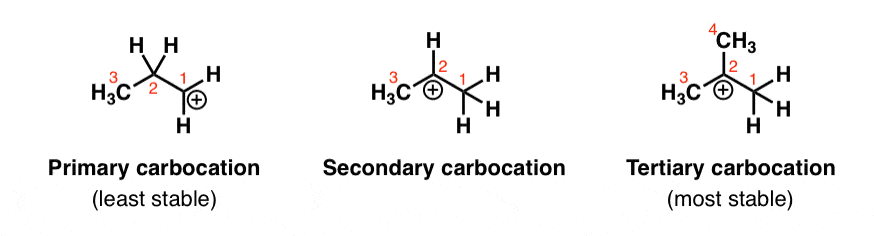
(It’s also worth pointing out that carbocations are also stabilized by resonance, which allows the positive charge to be delocalized or “spread out” over a greater area on the molecule.)
3. If A Less Stable Carbocation Can Be Transformed Into A More Stable Carbocation Through The Migration Of A C-H Bond, Then A Rearrangement Is Possible
So what does this have to do with rearrangements? As it turns out, if a situation exists where an unstable carbocation can be transformed into a more stable carbocation. then a rearrangement is possible.
One rearrangement pathway where an unstable carbocation can be transformed into a more stable carbocation is called a hydride shift. Look at the diagram below.
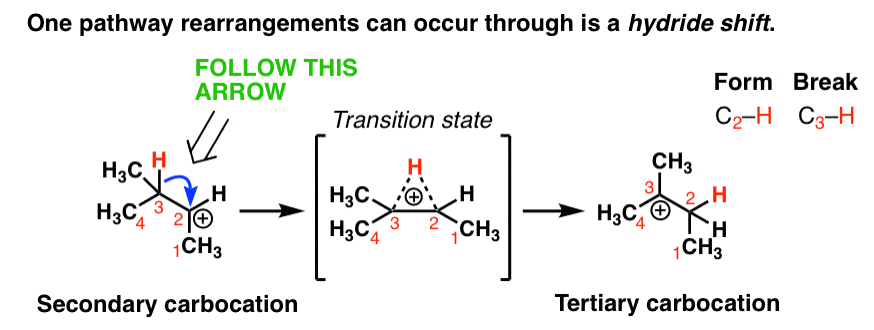
In this reaction we have a secondary carbocation on the left hand side. In this rearrangement reaction, the pair of electrons in the C-H bond is transferred to the empty p orbital on the carbocation. In the transition state of this reaction, there’s a partial C-H bond on C3 and a partial C-H bond on C2.
The transition state here is kind of like that split second in a relay race where one sprinter is passing the baton to another sprinter and they both have their hands on it.
Then, as the C2-H bond shortens and the C3-H bond weakens, we end up with a carbocation on C3 (a tertiary carbocation) in the product which is more stable.
Note that we only need one arrow to show this occurring!
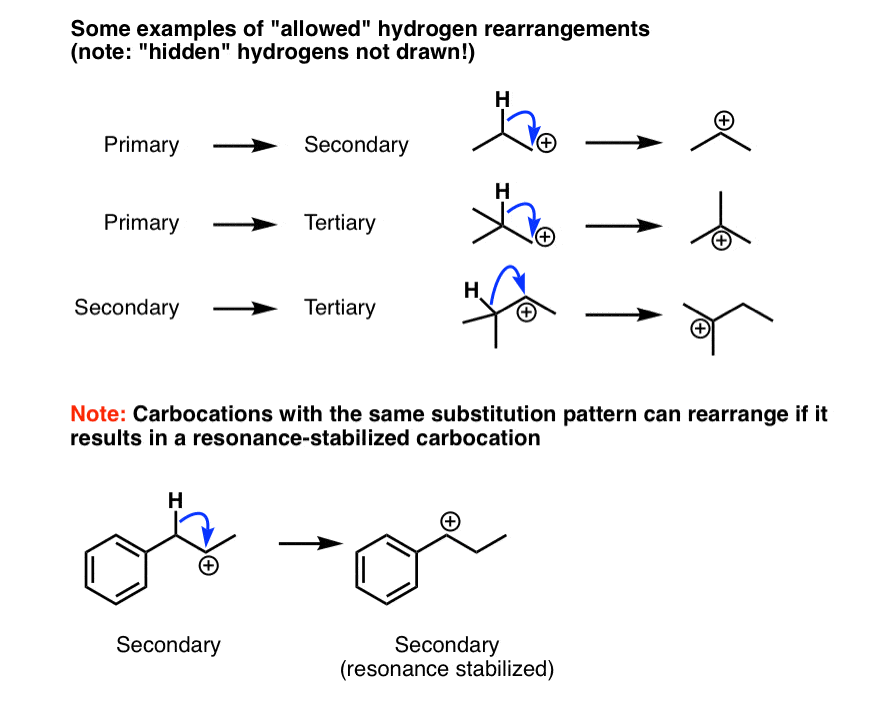
4. Examples Of “Allowed” Carbocation Rearrangement Reactions That Occur Through Hydride Shifts
Here are some examples of “allowed” rearrangement reactions. Notice how we’re always going from a less substituted carbocation to a more substituted carbocation. One exception is at the very bottom; the rearrangement is favorable because the new carbocation is resonance stabilized.
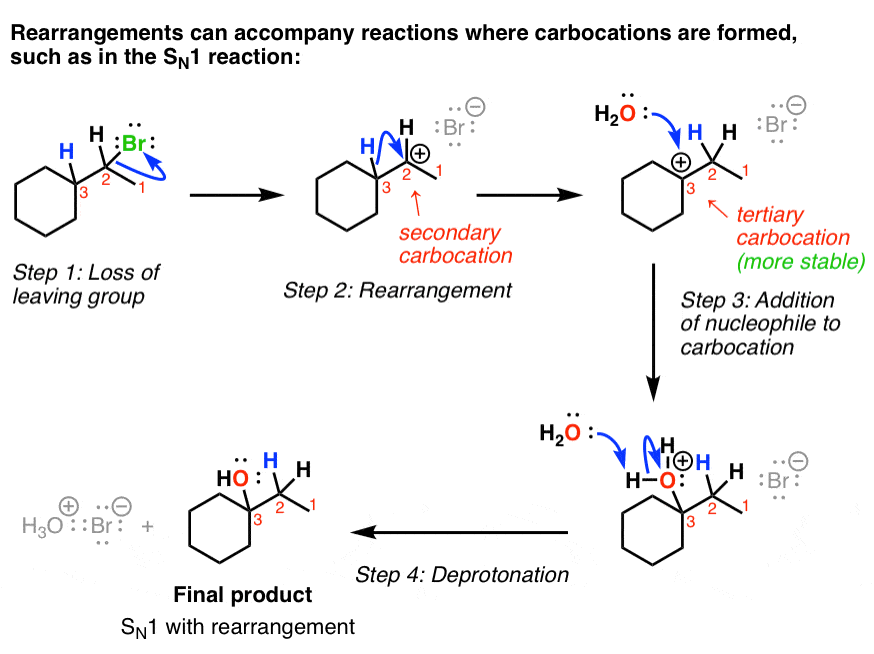
5. The SN1 Reaction With Hydride Shift: Arrow Pushing Mechanism
Now we’re ready to show how the rearrangement reaction occurs with the SN1. Recall that the first step in the SN1 is that the leaving group leaves to give a carbocation.
In the case below, the carbocation that is formed is secondary, and there’s a tertiary carbon next door. Therefore, a rearrangement can occur to give the more stable tertiary carbocation, which is then attacked by the nucleophile (water in this case).
Finally, the water is deprotonated to give the neutral alcohol. So this is an example of an SN1 reaction with rearrangement.
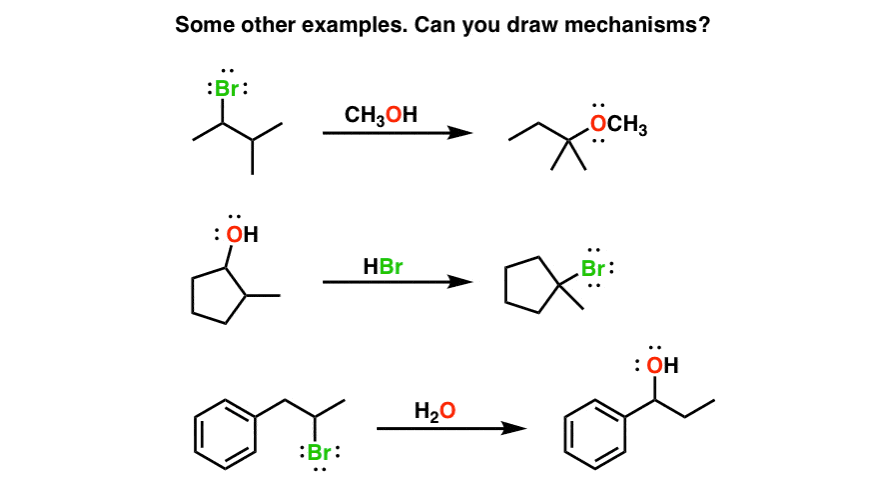
I’ve given some more examples of SN1 reactions with rearrangements below. See if you can draw the mechanisms! In the next post we’ll talk about a slightly different rearrangement pathway with substitution reactions.