“A-Values” For Substituted Cyclohexanes
“A-Values” are a numerical way of rating the bulkiness of substituents on a cyclohexane ring. The “A-Value” represents the difference in energy (in kcal/mol) between the cyclohexane conformation bearing the group in the equatorial position (more favored) and the cyclohexane conformation bearing the group in the axial position. The greater the “A-value” the higher the energetic preference for the equatorial position, and the more “bulky” the group is considered.
Table of Contents
- A Numerical Ranking of “Bulkiness” For Cyclohexane Substituents
- Ethyl (1.75 kcal/mol)
- Hydroxyl (OH) (0.87 kcal/mol)
- Br (0.43 kcal/mol)
- Isopropyl (2.15 kcal/mol)
- tert-Butyl (4.9 kcal/mol)
- Summary: “A-Values”
- Notes
- (Advanced) References and Further Reading
1. A Numerical Ranking Of “Bulkiness” For Cyclohexane Substituents
In the last post we saw that adding a methyl group to cyclohexane results in two chair conformers that are unequal in energy. We saw that the conformer where the methyl group was equatorial is the most stable, since it avoids destabilizing diaxial interactions (technically, gauche interactions) that are present in the conformer when the methyl group is axial.
We also said that experiments tell us that 1-methylcyclohexane exists as a 95:5 ratio of conformers at room temperature (favouring the more stable equatorial conformer) and by using the equation ΔG = –RT ln K we can calculate the energy difference, which turns out to be 1.70 kcal/mol.
The next logical question is this. What’s the energy difference for other groups? For example, what happens when we substitute ethyl (CH2CH3) for methyl ? Or OH ? Or Br ? Or tert-butyl ? How is the equilibrium affected?
In order to find this out, it’s necessary to set up some experiments that allow us to measure these numbers. However, it’s all been done for us, so we can now present the results.
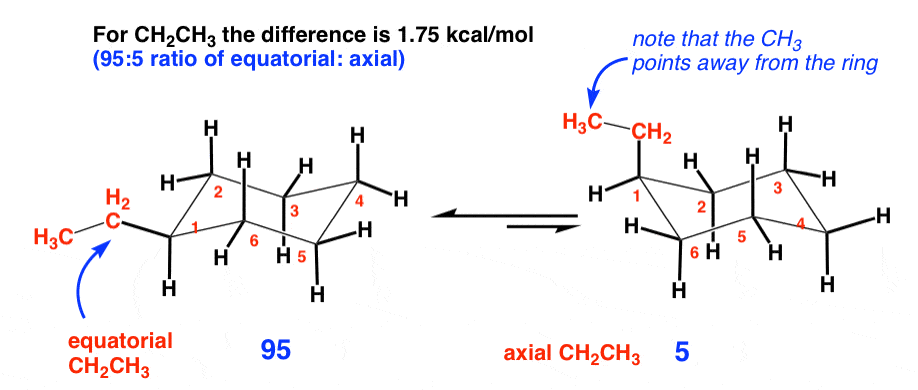
2. Ethyl (1.75 kcal/mol)
An ethyl group is one carbon larger than a methyl group. Naively, we might think that since it’s twice as long, it has twice as much steric hindrance, and the energy difference would be twice as big. However, the difference in energy is only 1.75 kcal/mol (compare to methyl at 1.70 kcal/mol). This is because the only significant diaxial interactions are with the CH2 group. The ethyl group can rotate such that the CH3 points away from the ring, where it does not lead to any significant increase in strain.
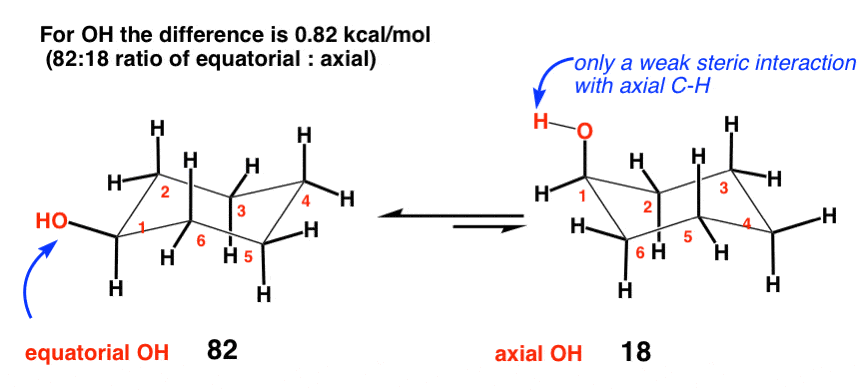
3. Hydroxyl (OH) (0.87 kcal/mol)
Given that oxygen has a larger atomic number than carbon, it’s not unreasonable to think that the OH group might be “bulkier” than carbon. When you think about the source of strain in CH3, however, you realize that it’s not necessarily the size of the carbon atom itself but the hydrogens of CH3 interacting with the axial hydrogens on the ring that lead to strain. Oxygen, having only one hydrogen, can always rotate such that the H is pointing away from the cyclohexane, thereby leading to very little in the way of diaxial interactions with the ring.
The value for OCH3 is even less (0.6 kcal/mol).
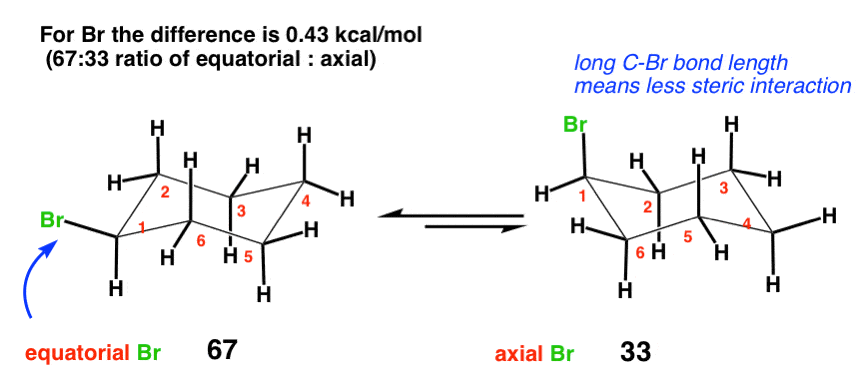
4. Br (0.43 kcal/mol)
Along similar lines one could be forgiven for thinking that Br, being such a heavy and large atom, might exert a large destabilizing influence when in the axial position. However, the difference is only 0.43 kcal/mol, less than that for OH. Why might this be? The answer here is bond length. The average C-Br bond is about 193 picometers in length (1.93 Angstroms) – compare this to 1.50 for the bond between C and CH3 in cyclohexane. The Br, being farther away, will thus have less interaction with the axial hydrogens. [Note – this A value of 0.43 is the average of two experimentally determined values [0.38 and 0.48]. ]
Interestingly, despite their great difference in size, the A values for Cl, Br, and I are all roughly similar (about 0.43 or so). This is because the increased size is balanced by the increased bond length – the halogens might be increasing in size along Cl <Br < I – but they are also getting farther away.
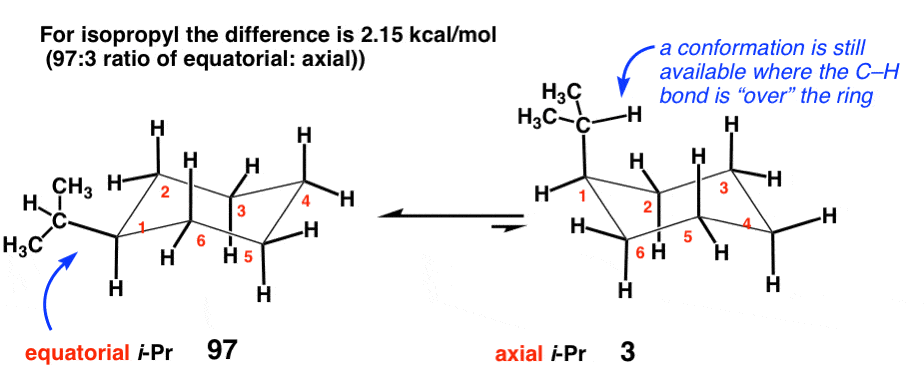
5. Isopropyl [-CH(CH3)2] (2.15 kcal/mol)
In contrast to ethyl, which has a secondary carbon attached to the ring, the isopropyl group represents a tertiary carbon attached to the cyclohexane. There is a relatively small but significant increase in strain to 2.15 kcal/mol . This is because the isopropyl group can still adopt a conformation where the C-H bond lies over the cyclohexane ring, which does not bring it into significant contact with the axial C-H bonds.
6. tert-butyl [-C(CH3)3] (4.9 kcal/mol)
This is the biggie. Look at the huge difference in energy between t-butyl (4.9 kcal/mol) and isopropyl (2.15 kcal/mol). What might account for that extra 2.7 kcal/mol in strain energy.
It helps to look at a figure.
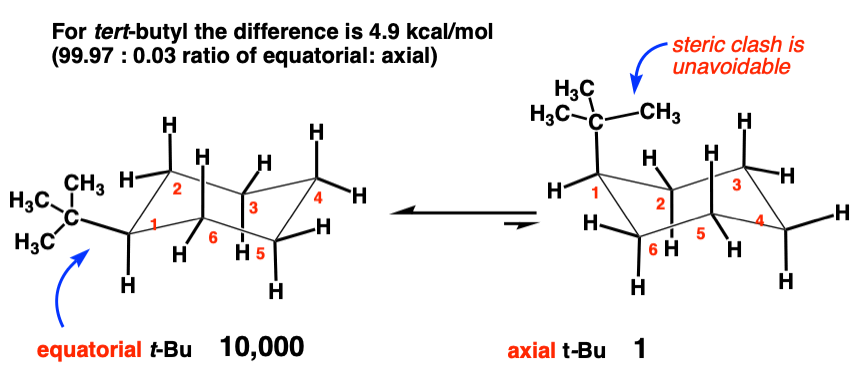
Notice how there’s no way to rotate the t-butyl group such that the methyl group is NOT pointing over the ring. A diaxial interaction between one of the methyl groups and an axial C-H is unavoidable. Axial t-butyl groups are strongly disfavoured.
What is the consequence of that value of 4.9 kcal/mol ? If we calculate the equilibrium constant K , it gives us a ratio of about 10,000 : 1 [accounting for only 2 significant figures here].
In other words, the concentration of axial t-butyl is 1/10,000 of that of equatorial t-butyl.
This value is so small that we often think of the t-butyl group as “locking” the cyclohexane ring in a position where the t-butyl is equatorial.
As we’ll see, this will have very important consequences for future reactions you’ll learn such as substitution and elimination, which can be sensitive to stereochemistry.
7. Summary: “A Values”
It’s nice to have some shorthand. For a mono-substituted cyclohexane, the energy difference between axial and equatorial conformers with a given substituent is known as its A-value.
For example, the A value of methyl is 1.70 , ethyl is 1.75, OH is 0.87, Br is 0.43, i-Pr is 2.15, and t-Bu is 4.9 .
A-values are useful because they are additive. We can use them to figure out the energy differences between di- and trisubstituted cyclohexanes, which is what we’ll talk about in the next post.
