Nucleophiles and Electrophiles, Nucleophilicity and Electrophilicity
All through the series on understanding where electrons are, and how they flow, we’ve been talking about how the basis of chemistry is that opposite charges attract and like charges repel, and that in reactions, electrons flow from “electron rich” areas to “electron poor” areas.
Today, we’ll officially give a name to the types of species that are considered “electron rich“ and “electron poor”.
They’re called nucleophiles and electrophiles.
Table of Contents
- A Nucleophile Is A Reactant That Provides A Pair Of Electrons To Form A New Covalent Bond
- An Electrophile Is A Reactant That Accepts A Pair Of Electrons To Form A New Covalent Bond
- Nucleophilicity” And “Electrophilicity” Refer To The Extent To Which A Species Can Donate Or Accept A Pair Of Electrons
- The Vast Majority Of Reactions You Will See Are Reactions Between A Nucleophile And An Electrophile
1. A Nucleophile Is A Reactant That Provides A Pair Of Electrons To Form A New Covalent Bond
Let’s start with “nucleophiles” (from “nucleus loving”, or “positive-charge loving”). A nucleophile is a reactant that provides a pair of electrons to form a new covalent bond.
Sound familiar? It should! This is the exact definition of a Lewis base.
In other words, nucleophiles are Lewis bases.
When the nucleophile donates a pair of electrons to a proton (H+) it’s called a Brønsted base, or simply, “base”.
Here are some examples of Lewis bases you’re probably familiar with.
As you can see, nucleophiles all have pairs of electrons to donate, and tend to be rich in electrons. [Moving ahead, there are actually three classes of nucleophiles you’ll meet in organic chemistry, but let’s focus on the simple examples for now.]
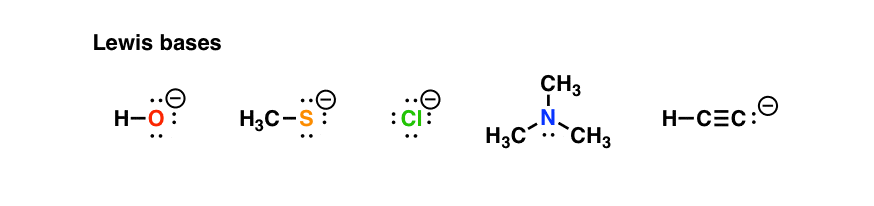 Example:
Example:
Let’s look at an example we’re familiar with: hydroxide ion.
When hydroxide ion donates a pair of electrons to an electrophilic atom (such as carbon here) to form a new covalent bond, it is acting as a nucleophile.
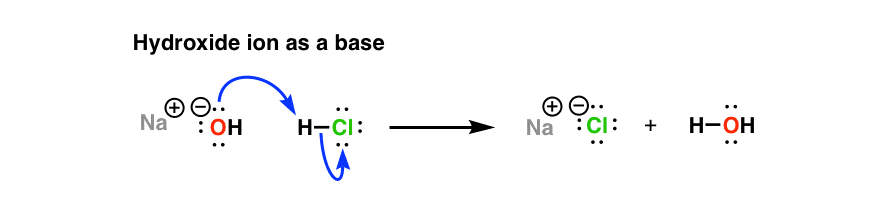
And as we’ve seen before, when hydroxide ion donates a pair of electrons to an (acidic) proton to form a new covalent bond, we say it’s acting as a “base“.
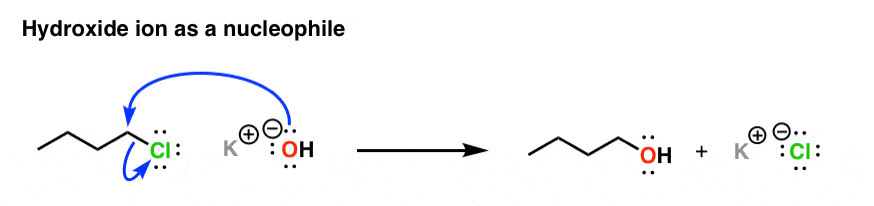
So species can be both nucleophiles and bases? Yes!!! In fact, the “basicity” we’ve been talking about is just a subset of “nucleophilicity” – the special case where the electrophile is a proton (H+)!
2. An Electrophile Is A Reactant That Accepts A Pair Of Electrons To Form A New Covalent Bond
Now let’s talk about electrophilicity (from “electron-loving”, or “negative-charge loving”). An electrophile is a species that accepts a pair of electrons to form a new covalent bond.
Again, this should sound familiar: this is the definition of a Lewis acid!
An electrophile that accepts an electron pair at hydrogen is called a Brønsted acid, or just “acid”.
Here are some examples of Lewis acids you’re familiar with.
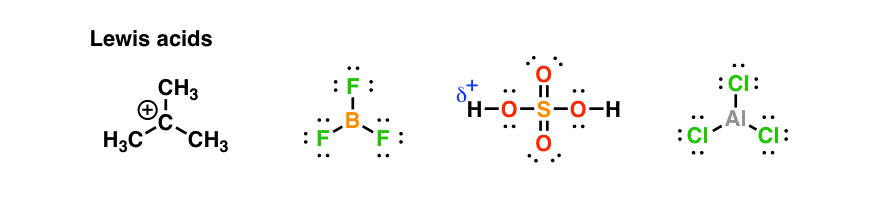
Just as species can be both nucleophiles and bases, other species can be both electrophiles and acids.
3. “Nucleophilicity” And “Electrophilicity” Refer To The Extent To Which A Species Can Donate Or Accept A Pair Of Electrons
We can vaguely define “nucleophilicity” as “the extent to which a species can donate a pair of electrons”. [There’s actually a more precise definition we’ll discuss in the next post, but this will do for now.]
Similarly, the extent to which a species can accept a lone pair of electrons is called “electrophilicity”.
And “acidity” is just a subset of “electrophilicity”.
4. The Vast Majority Of Reactions You Will See Are Reactions Between A Nucleophile And An Electrophile
Let’s go even further here: the vast majority of the reactions you’ll see (>95%) – will be reactions where a nucleophile donates a pair of electrons to an electrophile. Nucleophile attacks electrophile.
There are very few exceptions!
This is why understanding where electrons are, and how electrons flow is so important – because the electron richness (or poorness) of an atom (or molecule) determines its nucleophilicity or electrophilicity, which in turn determines its reactivity.
It’s not an exaggeration to say that nucleophilicity and electrophilicity are the fundamental basis of chemical reactivity. They are truly the yin and the yang of chemistry.
