As part 4 of the most important reactions you learn in org 1, (acid-base, substitution, and addition) here’s an introduction to the elimination reaction. This series requires that you understand how to read line diagrams (click for video introduction) as well as to understand what wedge-dash notation means (for showing molecules in 3D).
But otherwise as before, I’m not asking you to understand WHY any of these reactions happen. Just pay attention to the “plot” – that is, what bonds break and what bonds form, and recognize the pattern.
Let’s look at two simple cases. One important thing to keep in mind: although you see “Na OCH3” written here, the “Na+” (sodium) is not important for our purposes. It could alternatively be K+ (potassium) or Li+ (lithium). It’s just balancing the negative charge on the oxygen.
When you take an alkyl halide and add a strong base (such as NaOCH3 or NaOCH2CH3) a reaction occurs. See if you can recognize the bonds broken and formed.
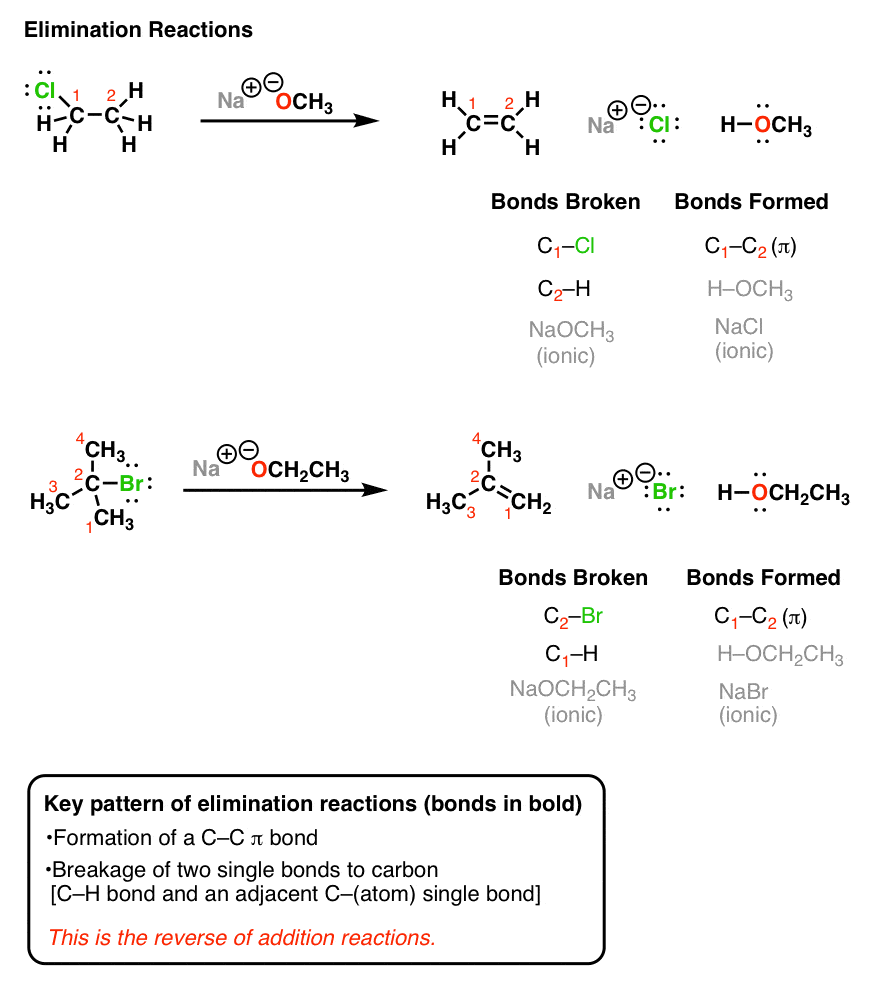
Hopefully you can see here that we are forming a new C-C double bond (π bond) and breaking two single bonds to carbon (in these cases, one of them is H and the other is a halide such as Cl or Br). We are also forming a new bond between H and the O. So in this respect, this reaction incorporates a pattern we have seen before – an acid-base reaction. Finally, we also form a salt in this reaction. Since we’re primarily interested in the organic product (that is, the one containing carbon), you might find that the salt byproduct is not written in some reaction schemes, but that doesn’t mean that it’s not there.
If you think about it, the three events just mentioned (formation of C-C π bond, breakage of two adjacent carbon sigma bonds) are in fact the exact opposite of the addition reaction we talked about last time.
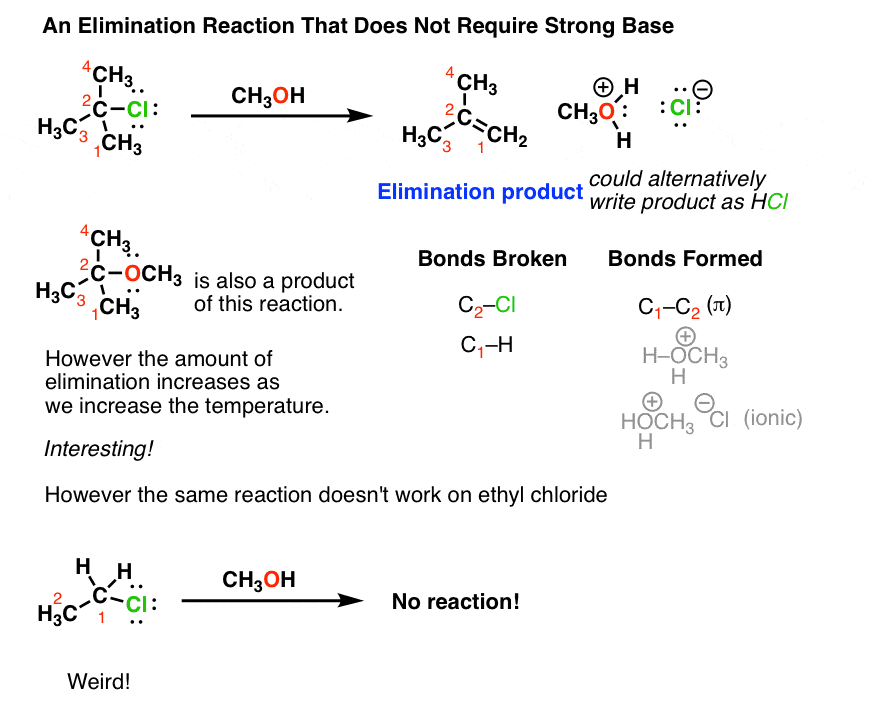
Although the first two examples used a strong base, there are cases where a strong base is not necessary. Like in the first reaction below. It’s still an elimination reaction, but in this instance we’re merely added methanol (CH3OH). Note that we’re still forming a C-C π bond and breaking two single bonds to carbon, but in this case our base is methanol (hence the formation of the salt, CH3OH2(+) Cl(-)). However if we take a molecule like ethyl chloride and treat it with methanol, we don’t obtain any elimination product at all. Weird.
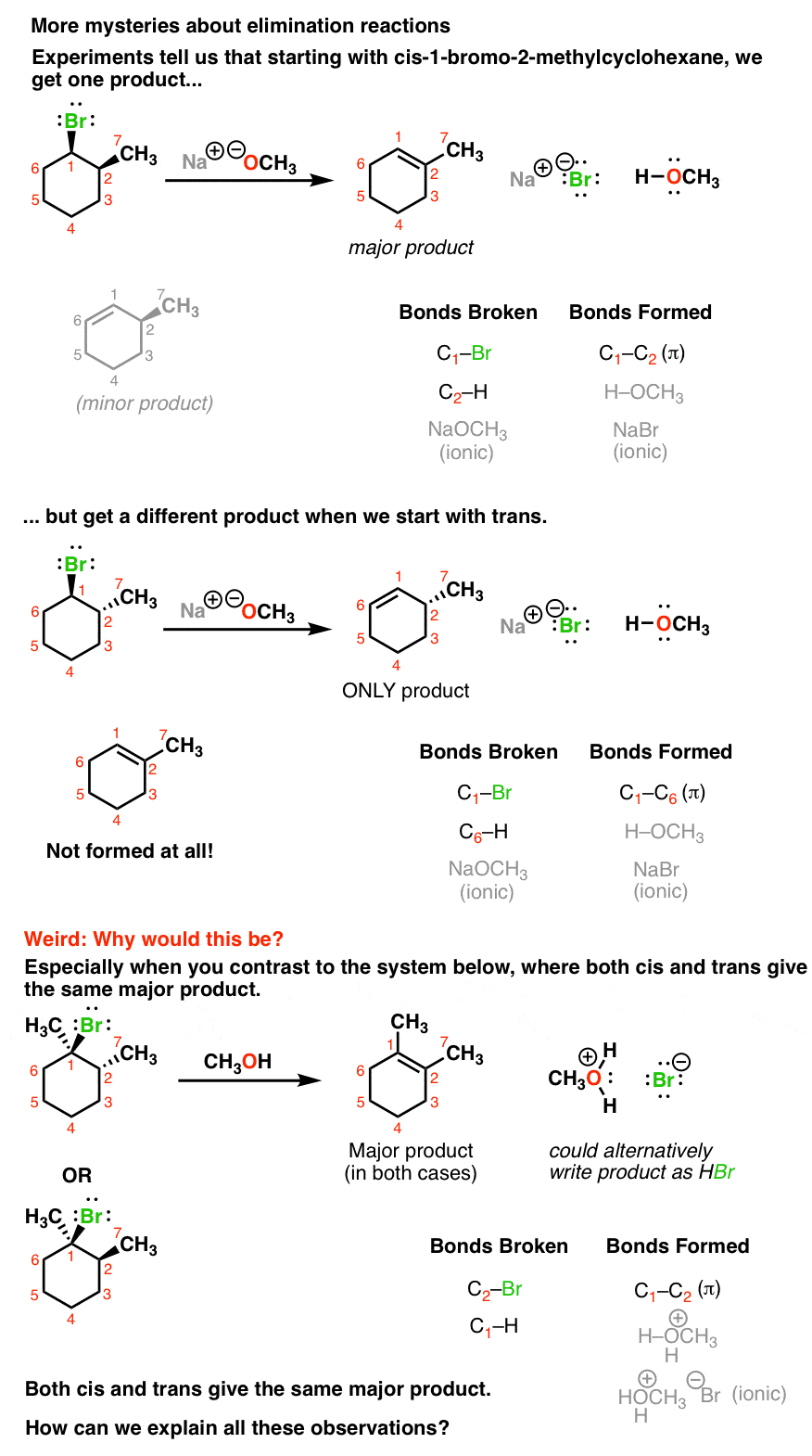
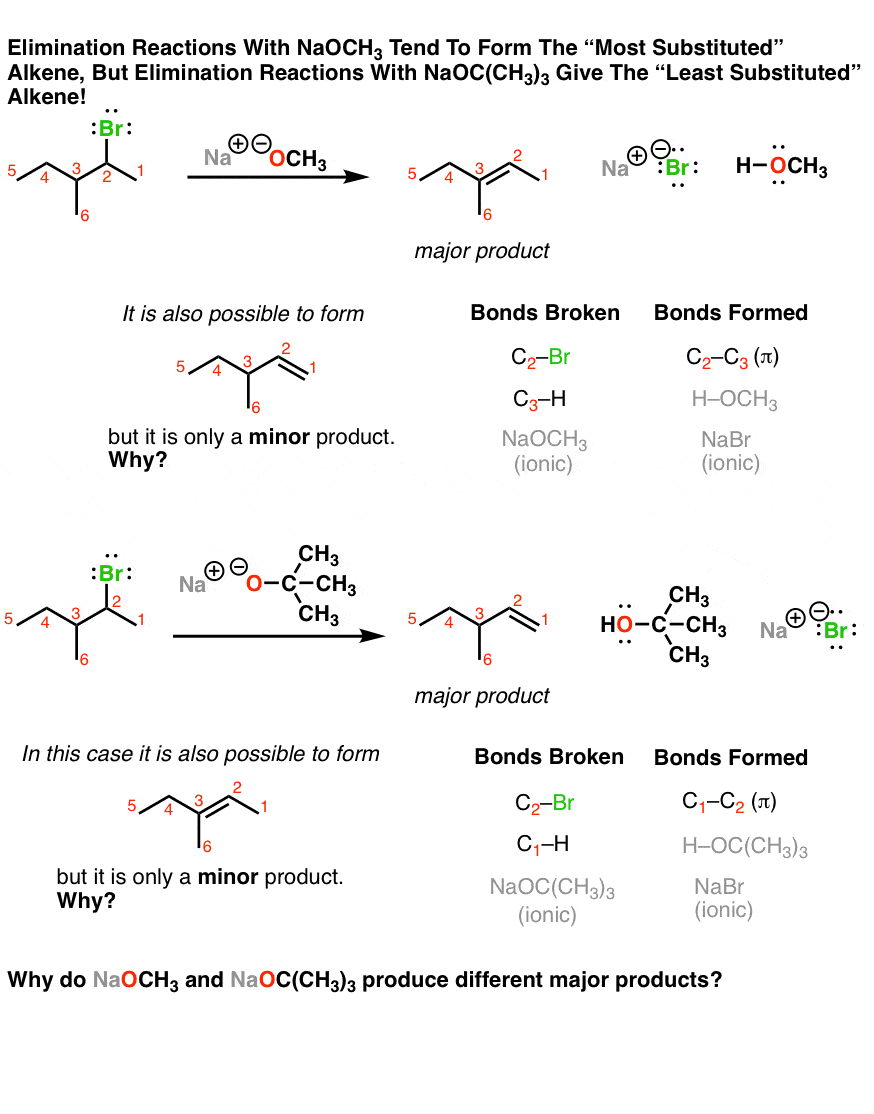
Now let’s look at what some experiments tell us. These are all elimination reactions, but they represent different twists on the same theme. More weirdness awaits when we make our alkyl halides a little bit more complicated. For instance when we take the alkyl halide below, we can theoretically get two different elimination reactions. When we use NaOCH3 as our base, the product with the double bond between C2 and C3 dominates. But when we use the base NaOC(CH3)3 as our base, the major product has the double bond between C1 and C2. What could be going on here?
Finally, elimination reactions also seem to be sensitive to the stereochemistry of the alkyl halides we use. For example, if we start with the first alkyl halide below (where the Br and the methyl group are cis), we obtain the major product where the double bond is between C1 and C2. There is also a minor amount of a product with the double bond between C1 and C6.
However, if we keep everything the same except use the trans alkyl halide, we ONLY obtain the product with the alkene between C1 and C6, and don’t obtain any of the C1-C2 alkene at all.
In time, we will talk about how to answer some of these questions. But for now, just being able to recognize elimination reactions is a good place to start.