Cycloalkanes: Two Key Consequences of The Fact That Hydrocarbons Can Form Rings
In the first few weeks of an organic chemistry class, we’ve learned that:
- Carbon can form up to four single bonds
- Carbon with four single bonds adopts a tetrahedral geometry (ideal bond angle: 109.5°)
- Compared to other atoms on the periodic table, [O, N, S, Si for example] carbon forms very strong bonds with itself and can therefore form stable chains
- Carbon-carbon single bonds can rotate freely, and the three-dimensional shapes that arise (“conformations”) can vary significantly in energy.
Nothing particularly strange about that so far. How about this:
- in addition to forming chains, carbon atoms can also form rings
In this first post on this series on cycloalkanes, we’ll discuss two key consequences of the fact that carbon can form rings, and then move forward with further posts in that vein.
Table of Contents
- Each Ring Decreases The Hydrogen Count By Two
- Cycloalkanes of Less Than 8 Carbons Cannot Be Turned Inside-Out Without Breaking Carbon-Carbon Bonds
- Notes
1. Each Ring Decreases The Hydrogen Count By Two
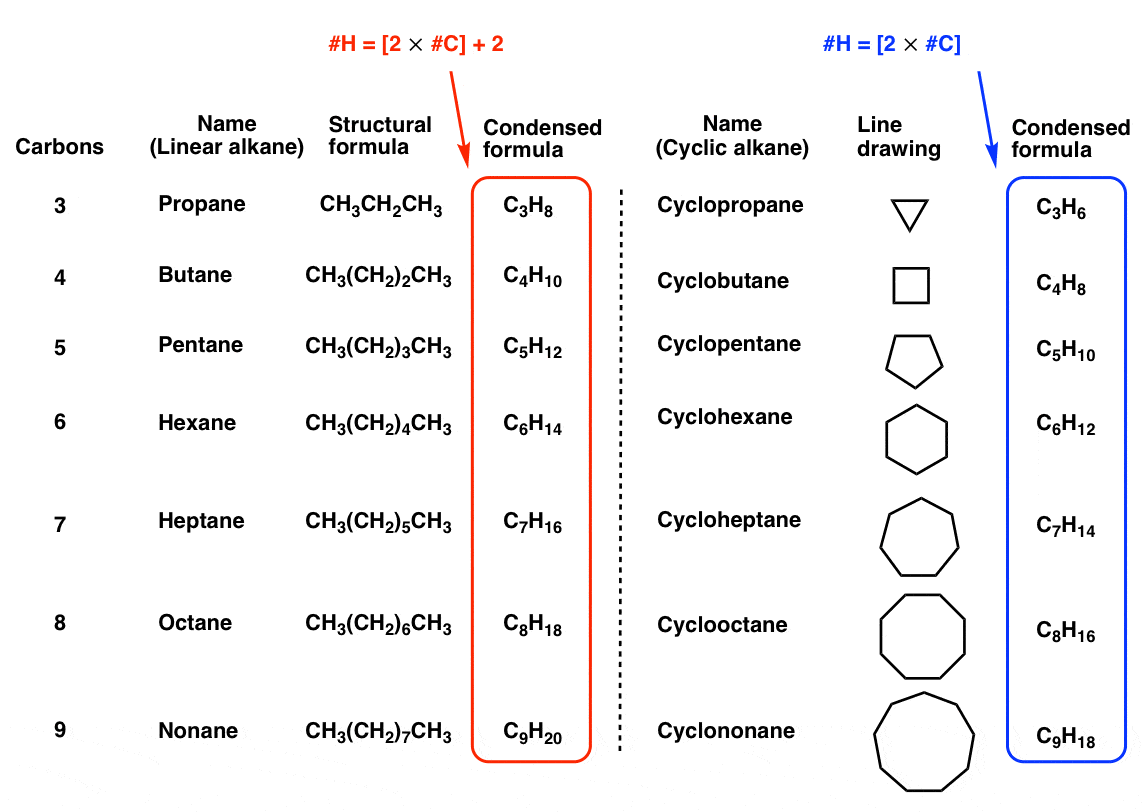
One of the first consequences of the fact that carbon can form rings can be found by comparing the condensed molecular formulae of linear alkanes with cyclic alkanes.
Notice how the formula of linear alkanes follows the pattern H = 2n + 2 (where “n” is the number of carbons) whereas the formula for cycloalkanes follows the pattern H = 2n.
Just by forming a ring, the number of hydrogens decreases by two!
By the way, every successive ring decreases the hydrogen content by two – the bicyclic molecules below follow each follow the pattern #H = 2n –2.
And the tricyclic molecule follows the pattern #H = 2n –4 .
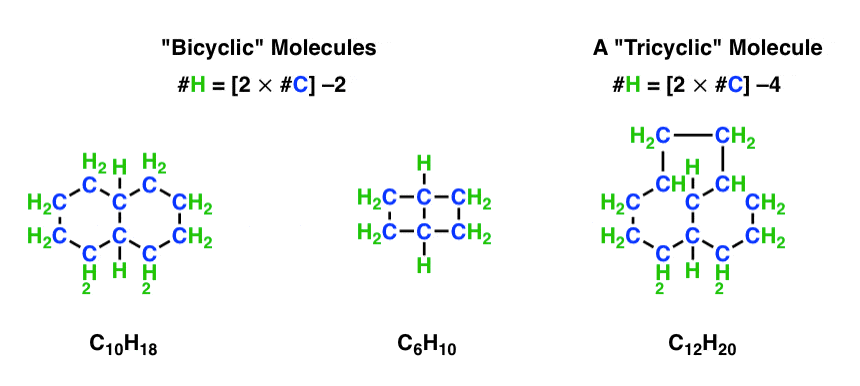
[Bonus Q – how many hydrogens would be in a tetracyclic molecule with 20 carbons (and no multiple bonds)? ]
Why does this matter? As you’ll see later, we’ll be able to use the fact that each ring decreases the hydrogen count of the molecule by 2 to help us deduce the structures of unknown compounds in some cases. It can be a small clue, but an important one nonetheless. [A look ahead – Degree of Unsaturation]
2. Cycloalkanes Of Less Than 8 Carbons Cannot Be Turned Inside-Out Without Breaking Carbon-Carbon Bonds
There’s a second interesting observation with cycloalkanes that we’ll talk about in much greater detail next time, but is important to get out of the way because it’s often overlooked. See how there’s that empty space in the middle of a cycloalkane ring? Many everyday household objects – belts, elastics, wristbands – can be easily turned inside out. Can we do the same with cycloalkanes? What happens when we try to turn them inside out?
Because it’s much easier to show this rather than tell it, I made a quick video.
The bottom line is that cycloalkanes of less than 8 carbons cannot be turned inside out without breaking carbon-carbon bonds. >99% of the rings that you’ll see in Org 1 / Org 2 will fall into this category.
This has far-ranging consequences that we’ll talk about in the next post in this series, namely that it gives rise to “cis” and “trans” isomers that cannot be interconverted.
