A Shortcut For Determining The Hybridization Of An Atom In A Molecule
Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in Org 1.
For a given atom:
- Count the number of atoms connected to it (atoms – not bonds!)
- Count the number of lone pairs attached to it.
- Add these two numbers together.
- If it’s 4, your atom is sp3.
- If it’s 3, your atom is sp2.
- If it’s 2, your atom is sp.
(If it’s 1, it’s probably hydrogen!)
The main exception is atoms with lone pairs that are adjacent to pi bonds, which we’ll discuss in detail below.
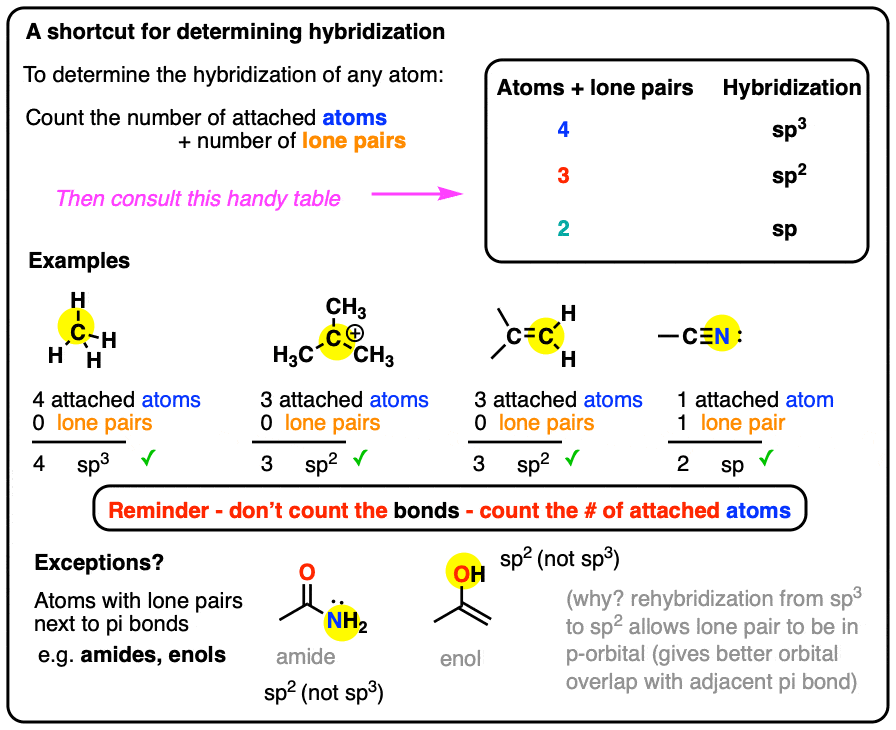
1. Some Simple Worked Examples Of The Hybridization Shortcut
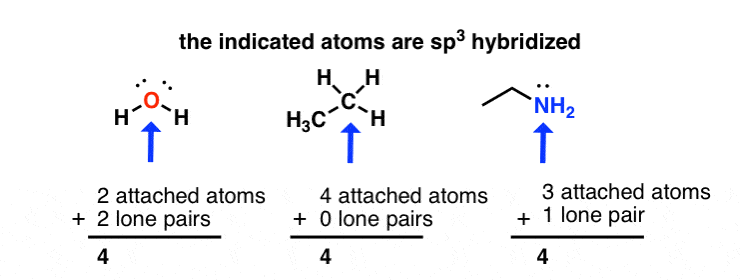
sp3 hybridization: sum of attached atoms + lone pairs = 4
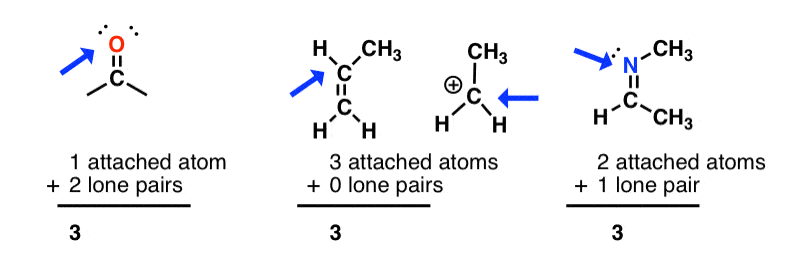
sp2 hybridization: sum of attached atoms + lone pairs = 3
sp hybridization: sum of attached atoms + lone pairs = 2
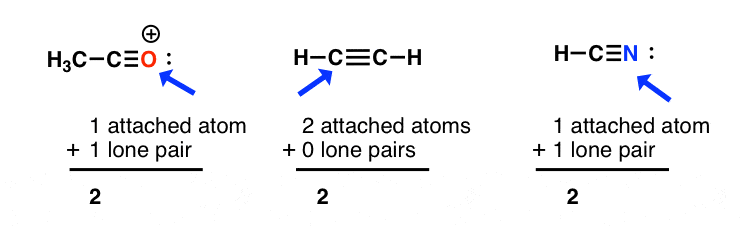
Where it can start to get slightly tricky is in dealing with line diagrams containing implicit (“hidden”) hydrogens and lone pairs.
Chemists like time-saving shortcuts just as much as anybody else, and learning to quickly interpret line diagrams is as fundamental to organic chemistry as learning the alphabet is to written English.
Remember:
- Just because lone pairs aren’t drawn in on oxygen, nitrogen, and fluorine doesn’t mean they’re not there.
- Assume a full octet for C, N, O, and F with the following one exception: a positive charge on carbon indicates that there are only six electrons around it. [Nitrogen and oxygen bearing a formal charge of +1 still have full octets].
2. How To Determine Hybridization Of An Atom: Two Exercises
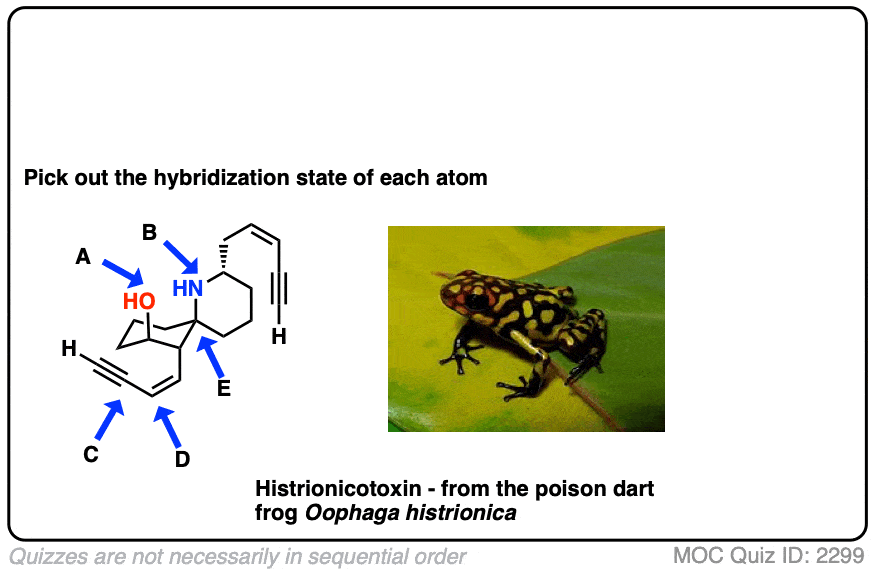
Here’s an exercise. Try picking out the hybridization of the atoms in this highly poisonous molecule made by the frog in funky looking pyjamas, below right.
[Don’t worry if the molecule looks a little crazy: just focus on the individual atoms that the arrows point to (A, B, C, D, E). A and B especially. If you haven’t mastered line diagrams yet (and “hidden” hydrogens) maybe get some more practice and come back to this later.]
 Click to Flip
Click to Flip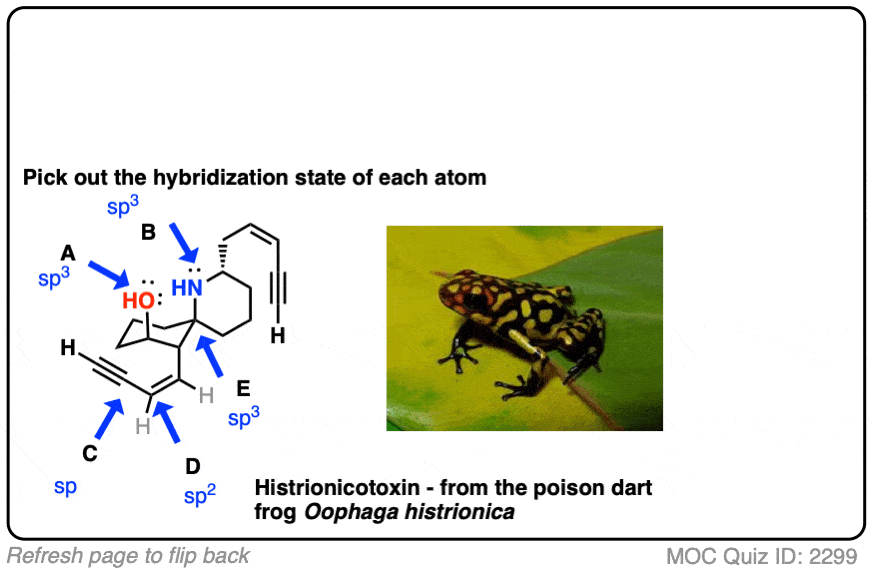
Here are some more examples.
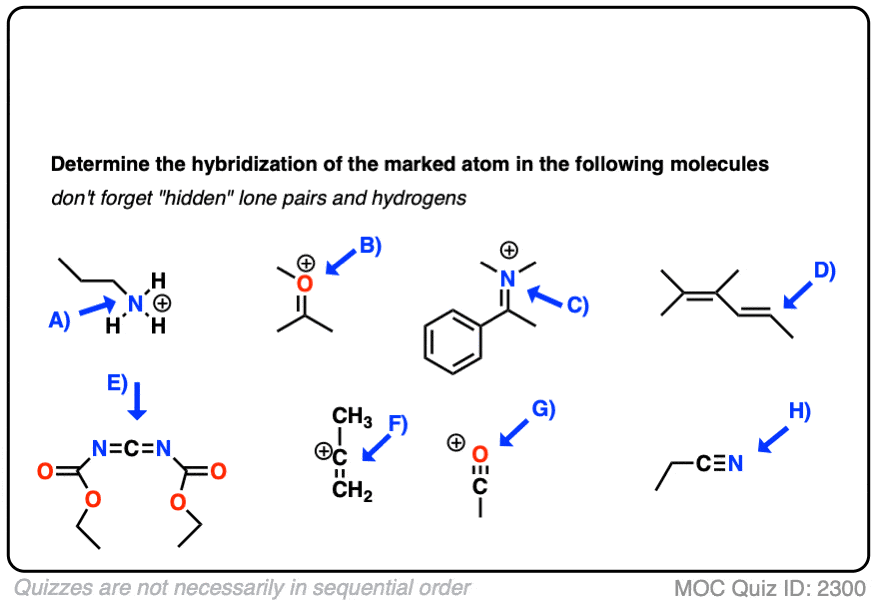 Click to Flip
Click to Flip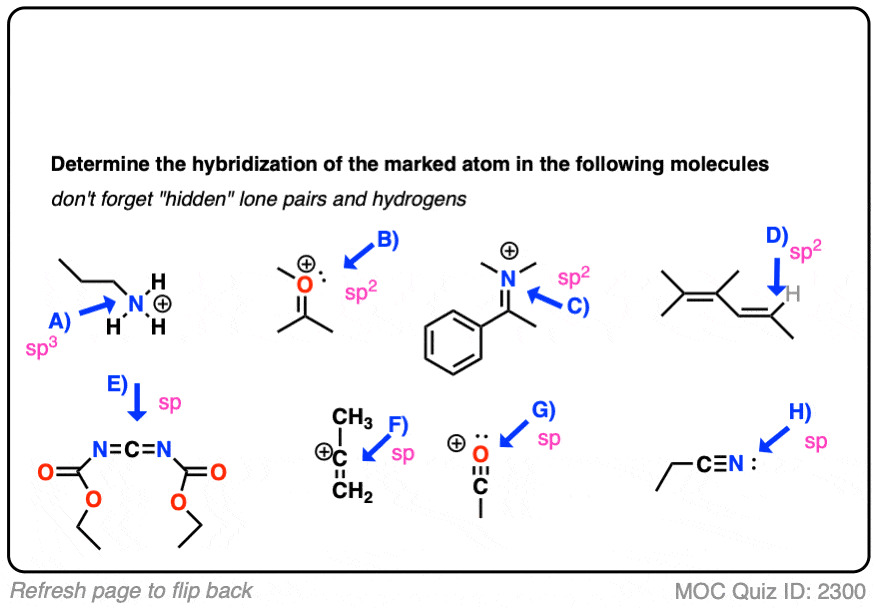
More practice quizzes for hybridization can be found here (MOC Membership unlocks them all)
3. Are There Any Exceptions?
Sure. Although as with many things, explaining the shortcut takes about 2 minutes, while explaining the exceptions takes about 10 times longer.
Helpfully, these exceptions fall into two main categories. It should be noted that by the time your course explains why these examples are exceptions, it will likely have moved far beyond hybridization.
Bottom line: these probably won’t be found on your first midterm.
4. Exception #1: Lone Pairs Adjacent To Pi-bonds
The main exception is for atoms bearing lone pairs that are adjacent to pi bonds.
Quick shortcut: Lone pairs adjacent to pi-bonds (and pi-systems) tend to be in unhybridized p orbitals, rather than in hybridized spn orbitals.
This is most common for nitrogen and oxygen.
In the cases below, a nitrogen or oxygen that we might expect to be sp3 hybridized is actually sp2 hybridized (trigonal planar).
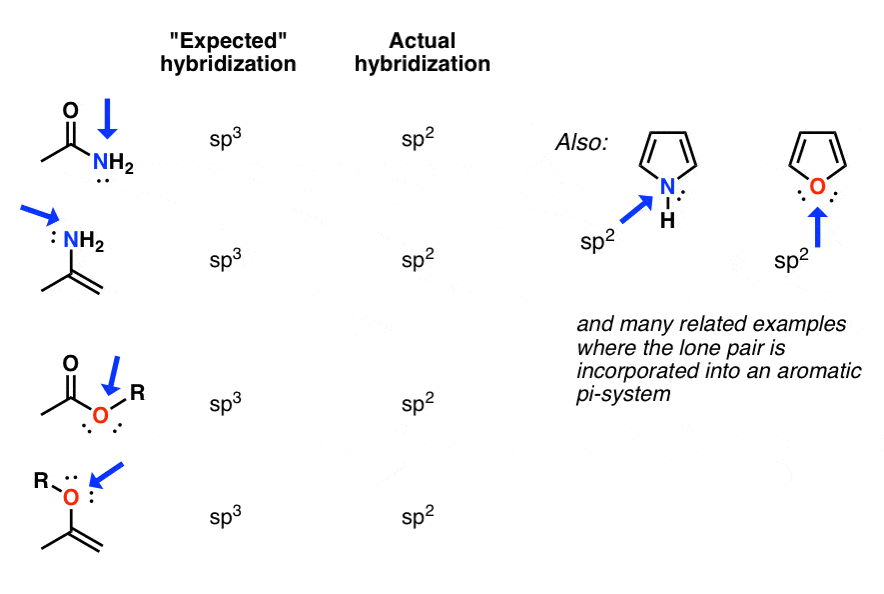
Why? The quick answer is that lowering of energy from conjugation of the p-orbital with the adjacent pi-bond more than compensates for the rise in energy due to greater electron-pair repulsion for sp2 versus sp3
[see this post: “Conjugation and Resonance“]
What’s the long answer?
5. Lone Pairs In P-Orbitals (Versus Hybrid Orbitals) Have Better Orbital Overlap With Adjacent Pi Systems
Let’s think back to why atoms hybridize in the first place: minimization of electron-pair repulsion.
For a primary amine like methylamine, adoption of a tetrahedral (sp3) geometry by nitrogen versus a trigonal planar (sp2) geometry is worth about 5 kcal/mol [roughly 20 kJ/mol].
That might not sound like a lot, but for two species in equilibrium, a difference of 5 kcal/mol in energy represents a ratio of about 4400:1] . [How do we know this? See this (advanced) Note 2 on nitrogen inversion]
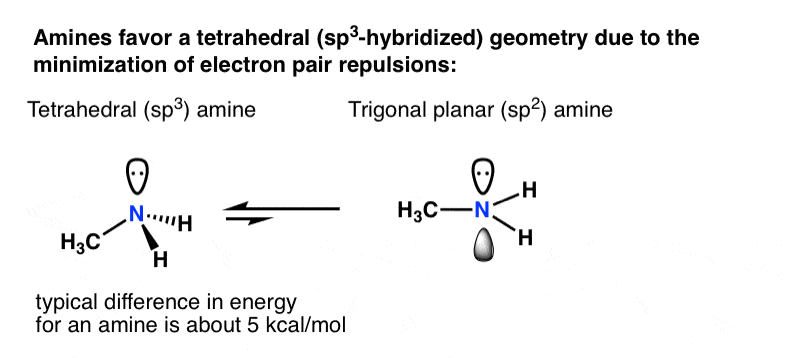
What if there was some compensating effect whereby a lone pair unhybridized p-orbital was actually more stable than if it was in a hybridized orbital?
This turns out to be the case in many situations where the lone pair is adjacent to a pi bond! The most common and important example is that of amides, which constitute the linkages between amino acids. The nitrogen in amides is planar (sp2), not trigonal pyramidal (sp3), as proven by x-ray crystallography.
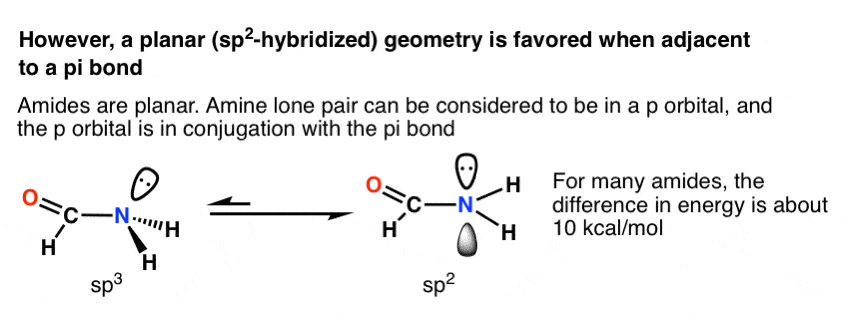
The difference in energy varies widely, but a typical value is about 10 kcal/mol favouring the trigonal planar geometry. [We know this because many amides have a measurable barrier to rotation a topic we also talked about in the Conjugation and Resonance post]
Why is trigonal planar geometry favoured here? Better orbital overlap of the p orbital with the pi bond vs. the (hybridized) sp3 orbital.
The drawing below tries to show how a change in hybridization from sp3 to sp2 brings the p-orbital closer to the adjoining p-orbitals of the pi bond, allowing for better orbital overlap. Better orbital overlap allows for stronger pi-bonding between the nitrogen lone pair and the carbonyl p-orbital, which results in an overall lowering of energy. 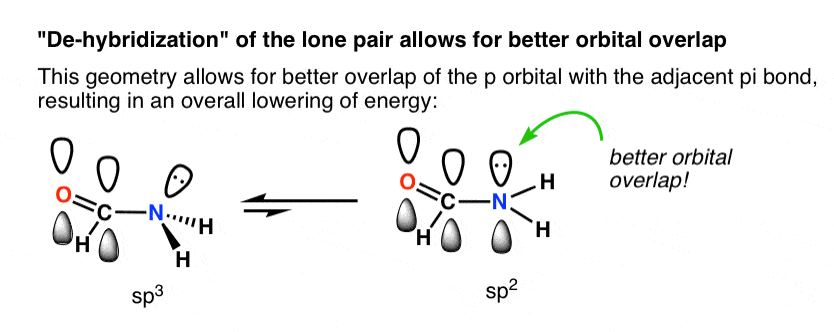
You can think of this as leading to a stronger “partial” C–N bond. Two important consequences of this interaction are restricted rotation in amides, as well as the fact that acid reacts with amides on the oxygen, not the nitrogen lone pair (!)
The oxygen in esters and enols is also also sp2 hybridized, as is the nitrogen in enamines and countless other examples.
As you will likely see in Org 2, some of the most dramatic cases are those where the “de-hybridized” lone pair participates in an aromatic system. Here, the energetic compensation for a change in hybridization from sp3 to sp2 can be very great indeed – more than 20 kcal/mol in some cases.
For this reason, the most basic site of pyrrole is not the nitrogen lone pair, but on the carbon (C-2) (!).
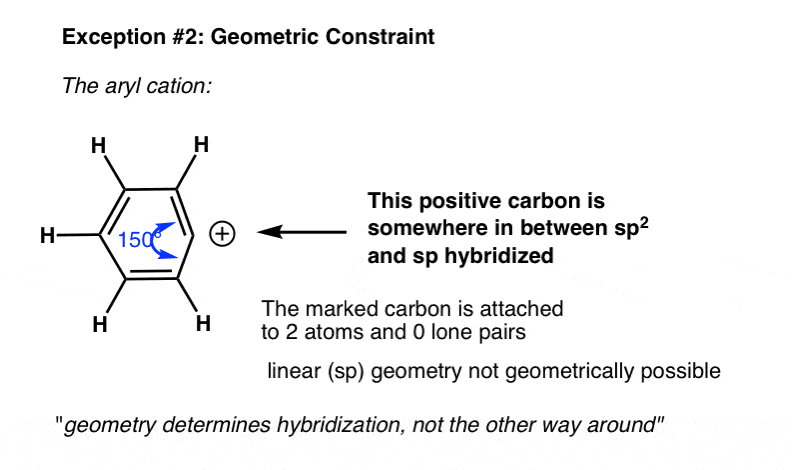
6. Exception #2. Geometric Constraints
Another example where the actual hybridization differs from what we might expect from the shortcut is in cases with geometric constraints. For instance in the phenyl cation below, the indicated carbon is attached two two atoms and zero lone pairs.
What’s the hybridization?
From our shortcut, we might expect the hybridization to be sp.
In fact, the geometry around the atom is much closer to sp2. That’s because the angle strain adopting the linear (sp) geometry would lead to far too much angle strain to be a stable molecule.