Description: Alkanes treated with chlorine gas (Cl2) and light (hv) or heat will be converted into alkyl chlorides.
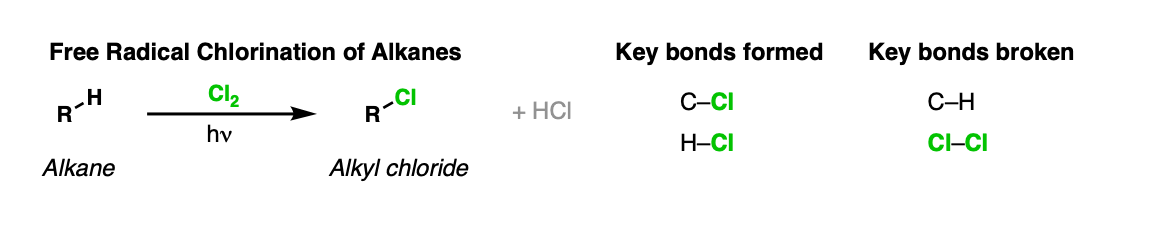
Notes: One equivalent of HCl is formed for every C–Cl bond that is formed. Also, the reaction is not particularly selective, which limits its usefulness (see below)
Examples:
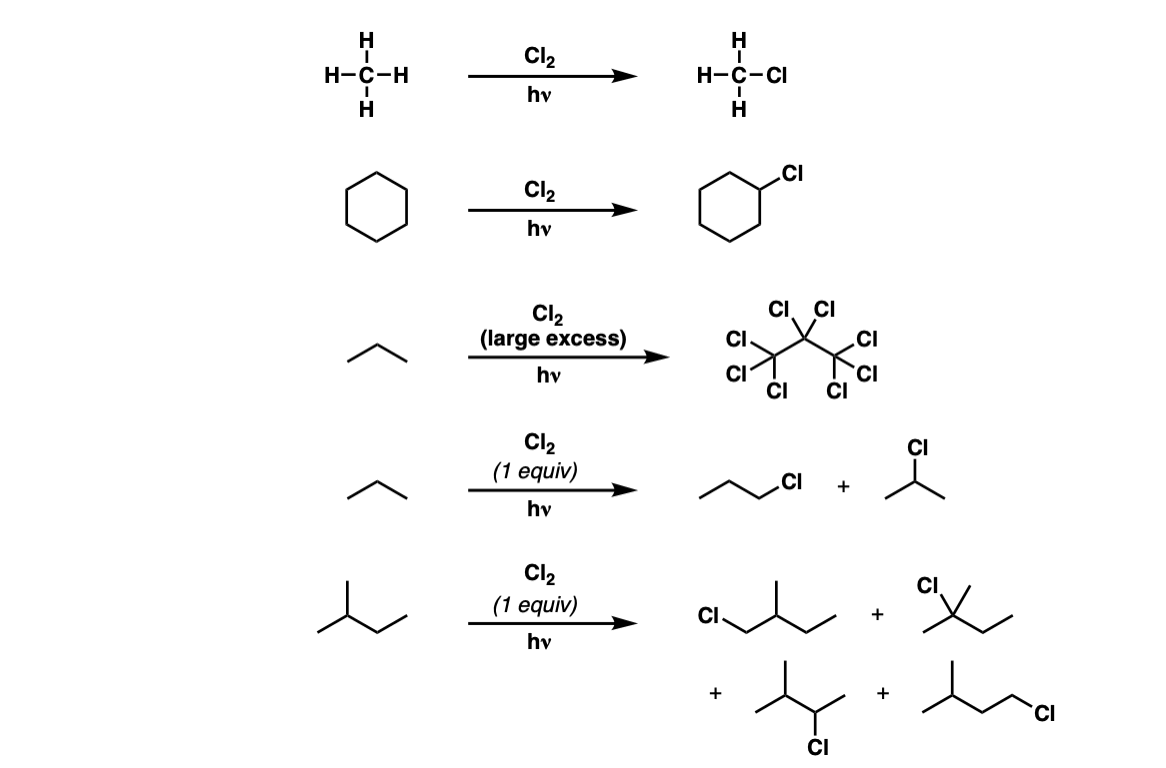
Notes: If this occurs at a stereocenter, a mixture of alkyl chlorides will be obtained. Also note that one equivalent of HCl is generated for every C–Cl bond that is formed.
Chlorine is not particularly selective, so the mixture of products will reflect statistics. For example in the third example (propane) there are six C–H bonds on the methyl groups, and two C–H bonds on the CH2 group, so the product mixture will be 3:1 favoring 1-chloropropane over 2-chloropropane.
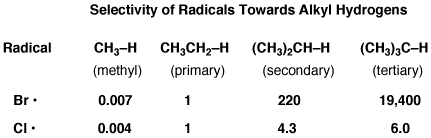
Here is a helpful table that lets you compare the selectivity of chlorine and bromine radicals on primary, secondary, and tertiary carbons:
Mechanism: When treated with light, Cl2 fragments to chlorine radicals (Step 1, arrow A). At any given time only a small amount of these radicals are present. There’s lots of leftover Cl2! Remember that, as it comes up in Step 3. Chlorine radical then abstracts a hydrogen from the alkane, leaving behind the alkyl radical (Step 2, arrows B and C). The alkyl radical then reacts with Cl2, giving the alkyl chloride (Step 3, arrows D and E) and a chlorine radical, which can be recycled to perform Step 2 with another equivalent of the alkane.
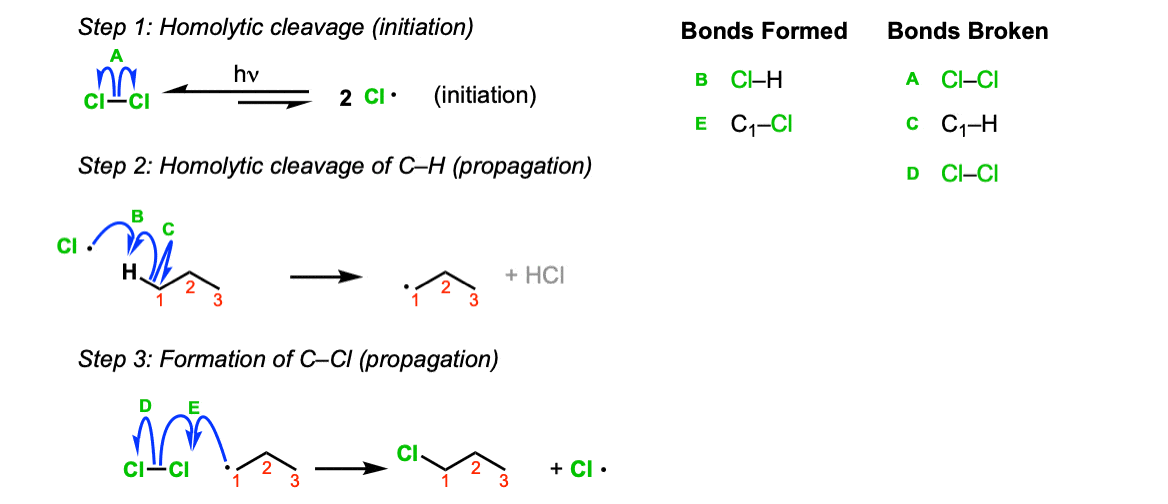
Notes: A common mistake is to show the carbon combining with Cl• in the second step! That’s not a propagation step, that’s termination!

