The Role of The Substrate In Substitution & Elimination Reaction: SN2 vs E1/SN1
- Deciding whether a reaction is SN1/SN2/E1/E2 first of all requires understanding the bonds that form and break in each of these four reactions and the key features of their mechanisms (Review here – SN1 / SN2 / E1 / E2)
- Primary, secondary, tertiary (and methyl) carbons attached to good leaving groups (such as alkyl halides and sulfonates) are good substrates for SN1/SN2/E1/E2 reactions. If this is not clear, review here
- If the alkyl carbon is primary, the lack of steric hindrance makes SN2 likely and the lack of carbocation stability makes SN1 and E1 unlikely. The main exception is with strong, bulky bases (E2).
- If the alkyl carbon is tertiary, it will not be SN2. More information is required to determine exactly which reaction will happen. Depending on the identity of the base (strong or weak) it may be SN1/E1 (weak base) or E2 (strong base)
- Secondary alkyl halides can potentially undergo all four reactions and require analyzing the nucleophile / base. (See here) as well as possibly the solvent and temperature.
- Alcohols can also be substrates for SN1/SN2/E1/E2 reactions, but require acidic conditions to convert OH into a good leaving group (OH2+ ). Basic conditions convert alcohols into alkoxides (RO– ) which are good nucleophiles.
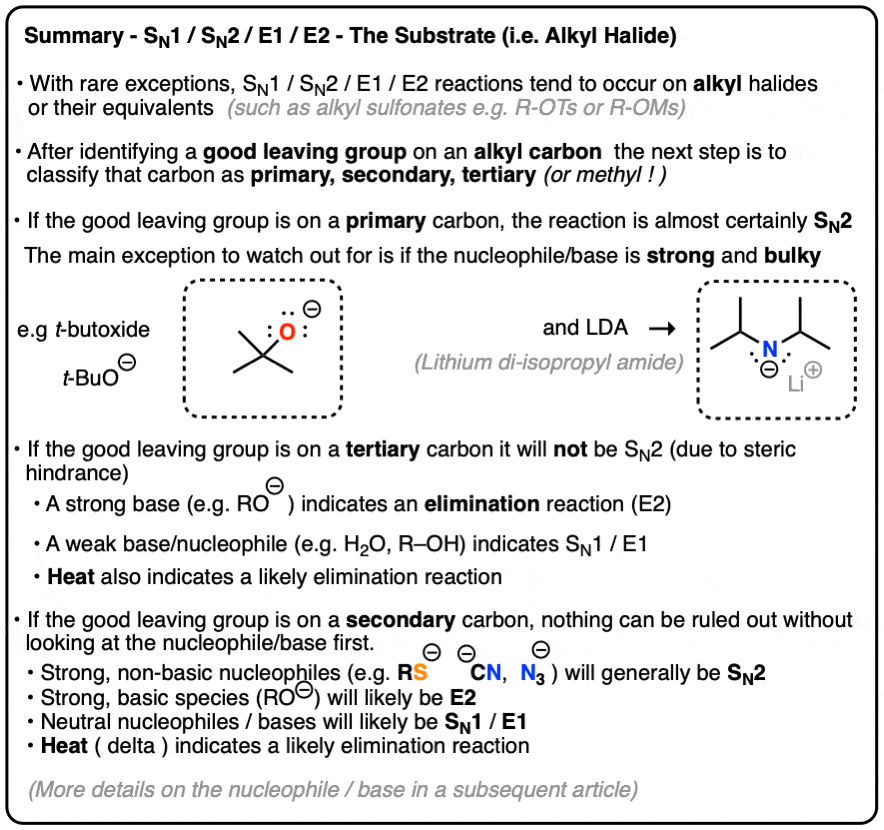
Table of Contents
-
- Step One: Determine If The Substrate is Primary, Secondary, or Tertiary
- Let’s Look At Some Example Reactions
- So The Substrate is Primary. Now What?
- What if It’s Tertiary?
- The Problem Child: Secondary Alkyl Halides
- A Look Ahead – Evaluating Nucleophile/Base, Solvent, Temperature
- Some Examples With Alcohols
- Summary
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. First Off: Determine If The Substrate Is Primary, Secondary or Tertiary
What’s the very first step in deciding whether a reaction goes through SN1, SN2, E1 or E2?
- The very first thing is to identify a good leaving group such as Cl, Br, I, or OTs/OMs! (For a refresher, see this previous post – How To Tell Where Substitution and Elimination Reactions Will Happen)
- The other key thing to note is that with very few exceptions, substitution and elimination reactions only happen on alkyl (i.e. sp3-hybridized) carbons.
So for our purposes, in order for SN1/SN2/E1/E2 to take place, we generally need to see an alkyl halide (or alkyl sulfonates, such as tosylates and mesylates – See post: Tosylates and Mesylates).
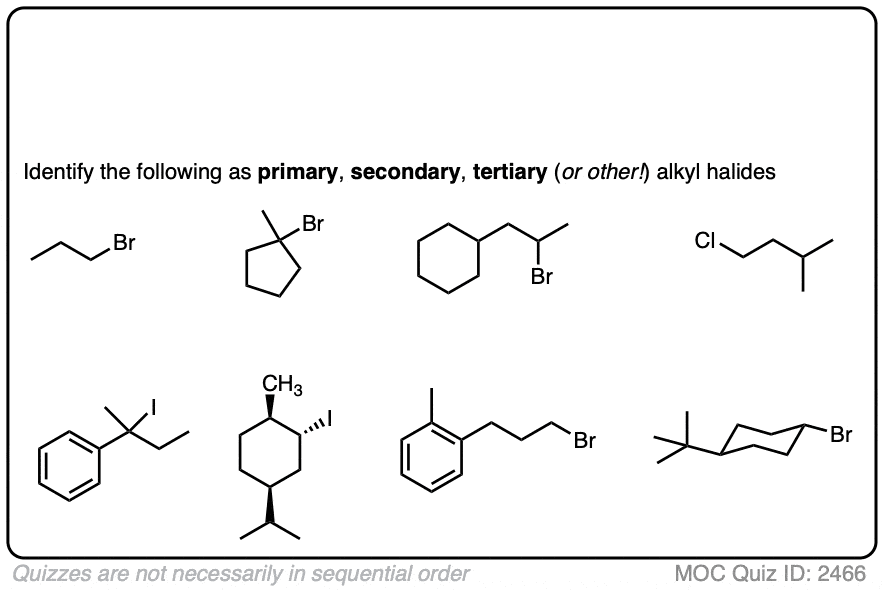
Once you’ve identified an alkyl halide, the next step is to determine whether it is primary, secondary, or tertiary.
Try it for yourself with this quick quiz!
 Click to Flip
Click to Flip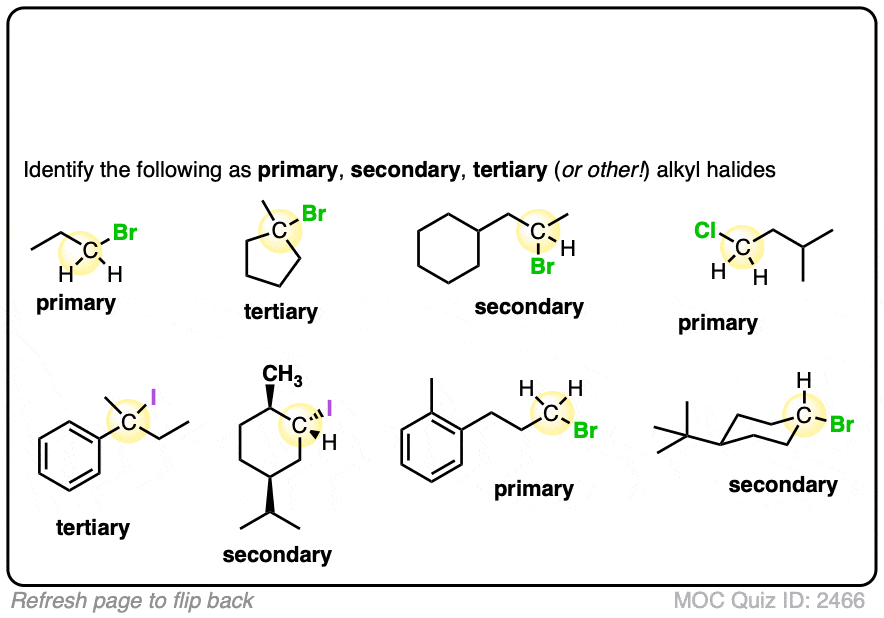
If these are difficult for you, then back up and have another look at this post (See article – Primary, Secondary, Tertiary, Quaternary in Organic Chemistry)
If this is too easy, then here’s a (slightly) more difficult set.
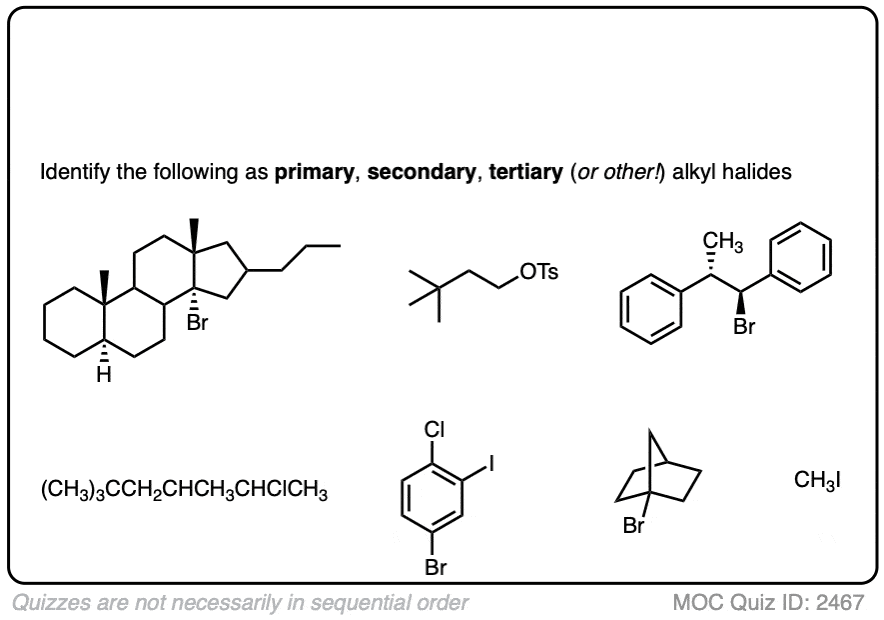 Click to Flip
Click to Flip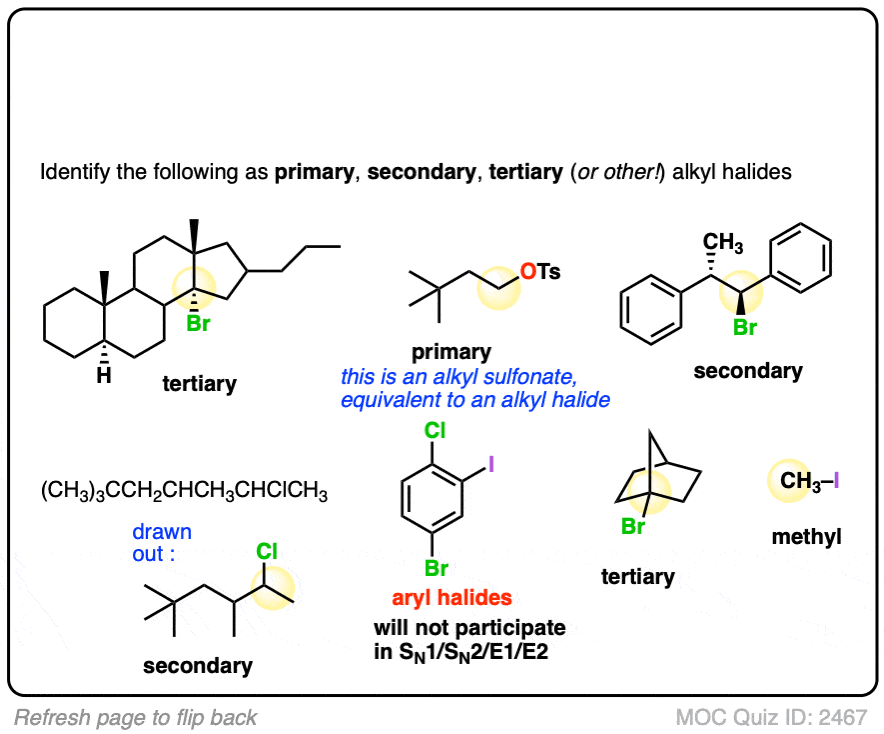
Note that sometimes these reactions are drawn out with reactions that won’t work. with the intention to test your understanding of mechanisms. If you see a question involving an alkenyl halide, your job is to figure out if it is put in there as a trick question, or if it qualifies as one of the (rare) exceptions to the “only alkyl carbons” rule. (The main exception is formation of alkynes via double elimination – see article)
2. Looking At Some Reactions
Once you can classify alkyl halides as primary, secondary, tertiary (or methyl!) the next step is to do it in the context of several reactions, where it’s not uncommon to see things drawn up a little unconventionally.
Before asking any other questions, look at the reactions below and see if you can identify potential substrates for SN1/SN2/E1/E2 reactions.
Let’s start with a fairly easy one. Identify primary, secondary, and tertiary:
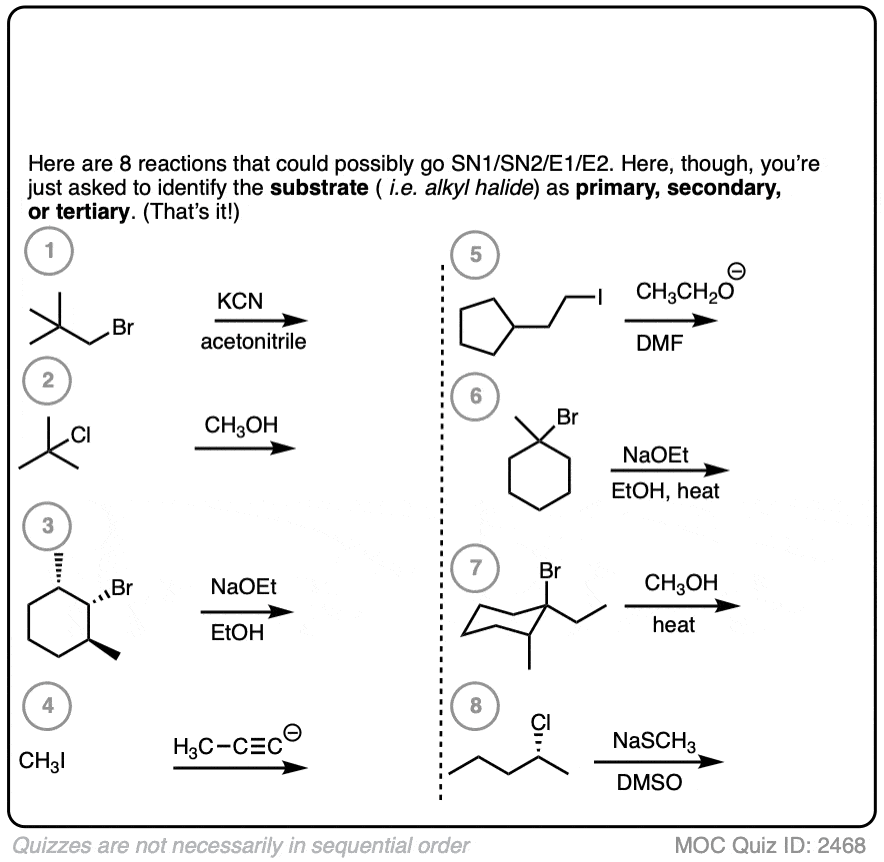 Click to Flip
Click to Flip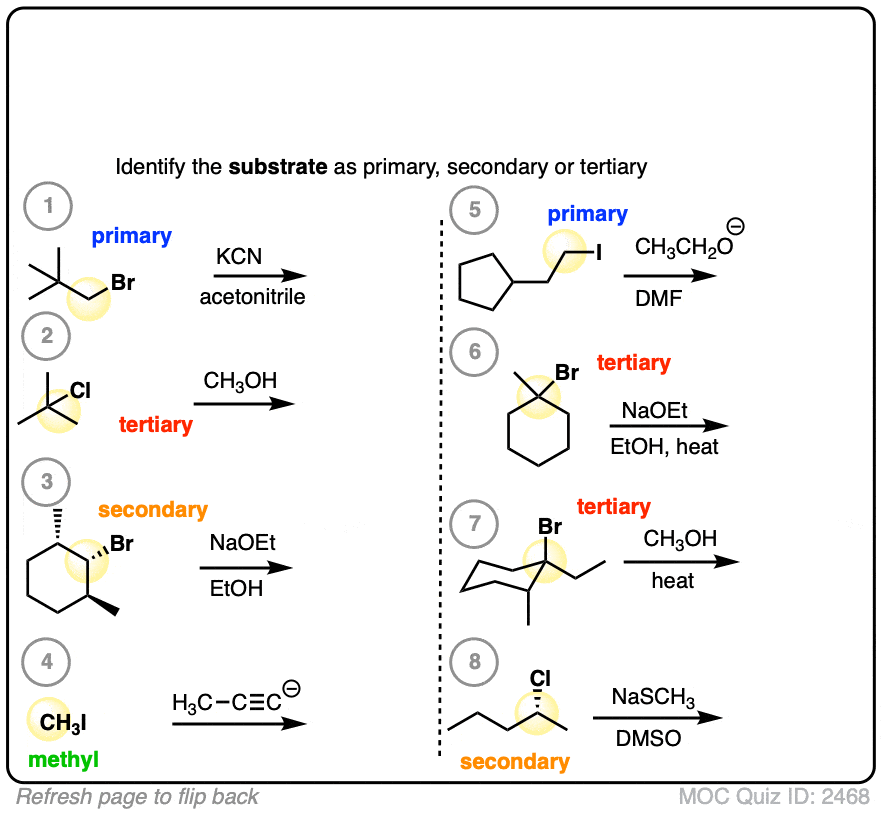
This one is slightly more difficult.
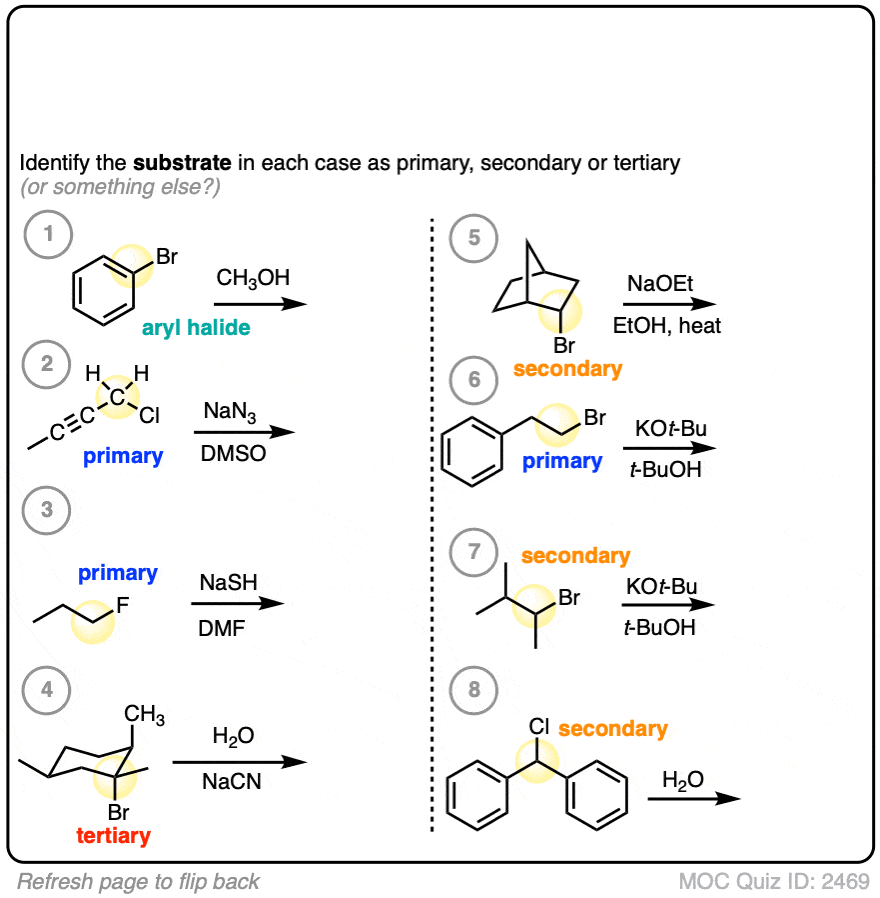 Click to Flip
Click to Flip
Finally, this has some harder examples.
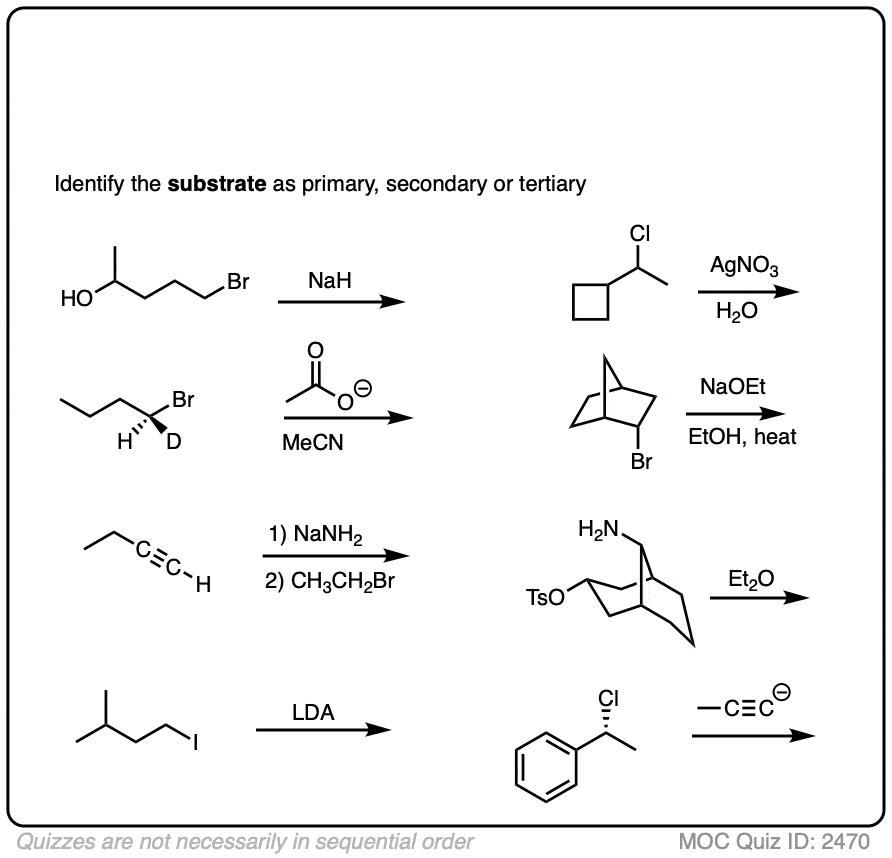 Click to Flip
Click to Flip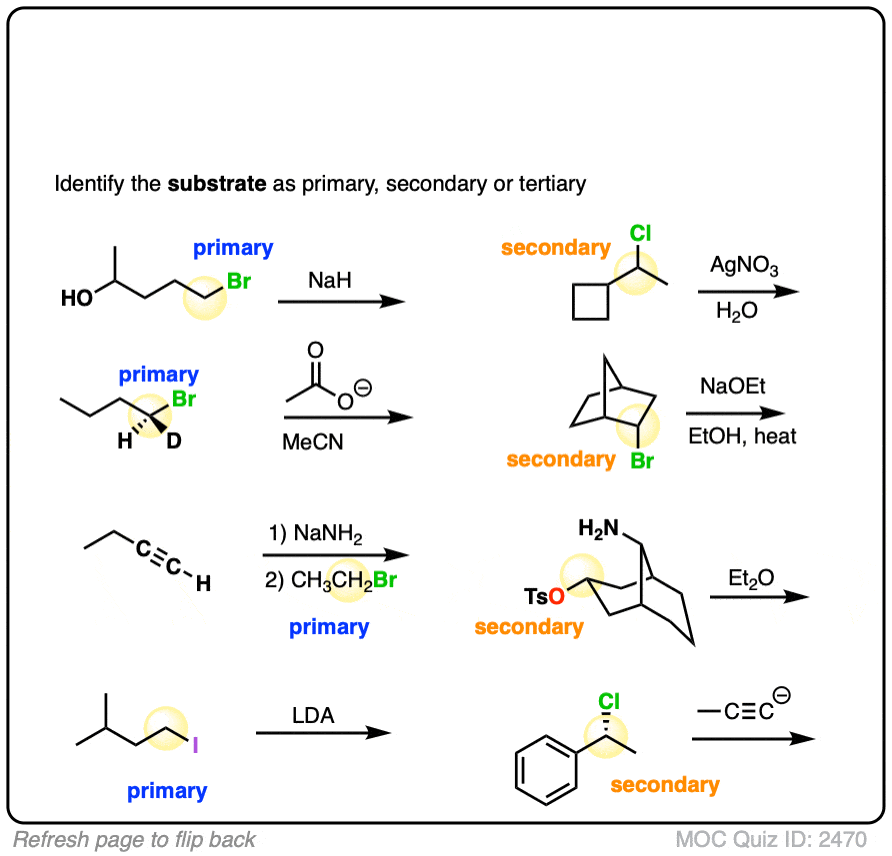
If you’ve looked at the reaction and found a primary, secondary, tertiary or methyl halide (or tosylate/mesylate, but not fluoride), then you are potentially looking at a substitution or elimination reaction.
If there’s no alkyl halide, then it’s very likely there’s no substitution or elimination reaction!
3. So The Alkyl Halide Is Primary – Now What?
All right. Let’s say we’ve identified our alkyl halide as primary.
Now what do we do?
We can make a confident prediction. When the substrate is primary (or methyl), expect SN2
These reactions will almost certainly not be SN1 or E1. [Note 1] That’s because the first step of SN1/E1 is loss of a leaving group to form a carbocation, and primary carbocations tend to be unstable. (See article: Carbocation Stability)
The next step is to predict the product. To do this, we:
- look for a good nucleophile, then
- apply the key pattern for the SN2 reaction (form C-Nu, break C-LG)
- invert the chiral center if there is one (rare for primary)
Also, it might help to recall the identities of common polar aprotic solvents such as DMF, DMSO, and acetonitrile (MeCN). These will affect the rate of substitution reactions but they are not nucleophiles. (See article – All About Solvents)
Now let’s put it into action. Assuming these reactions are SN2, draw the products.
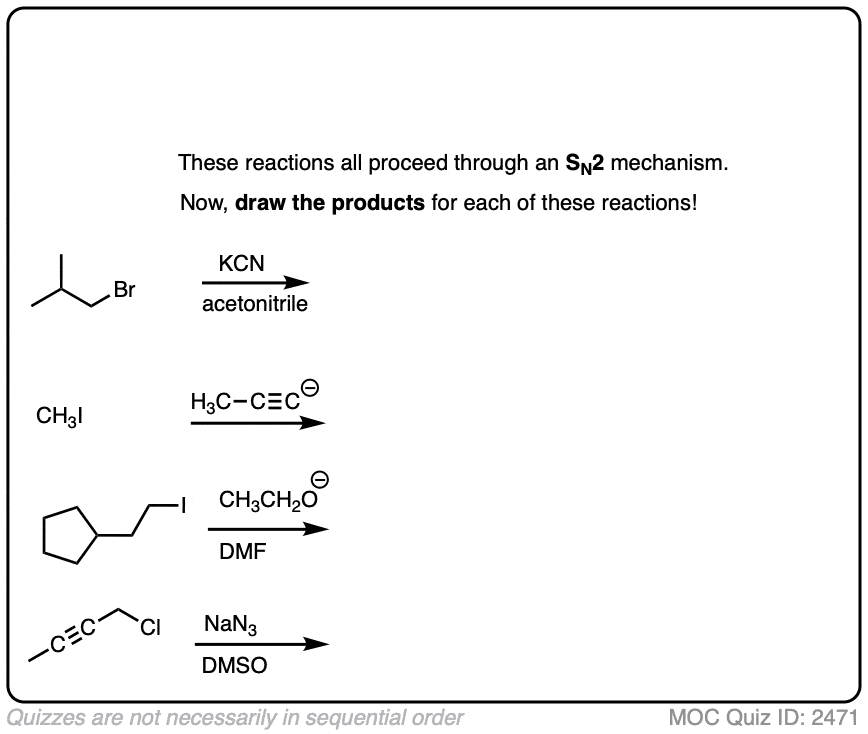 Click to Flip
Click to Flip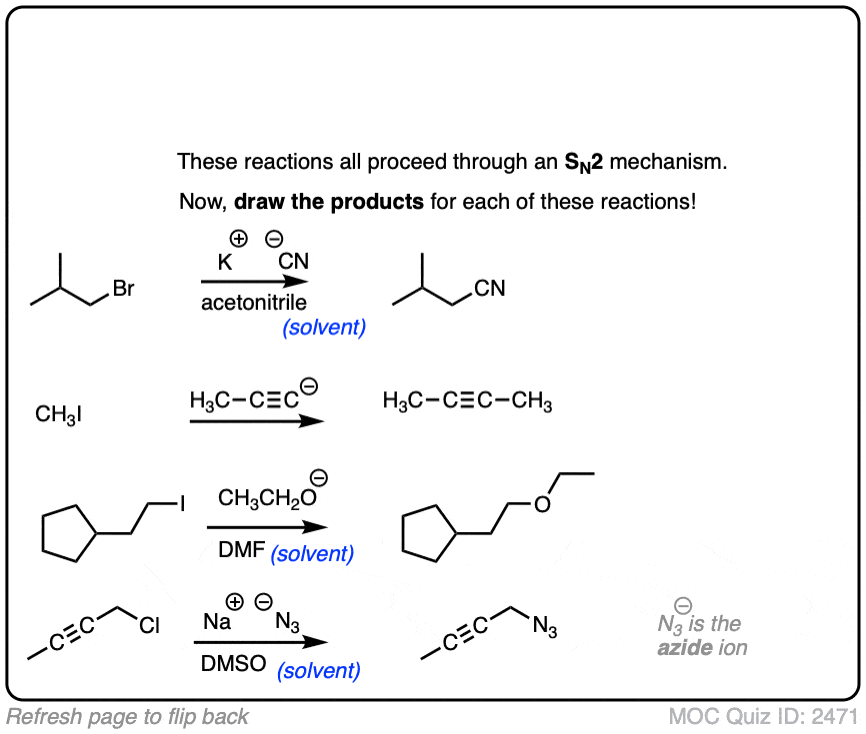
These examples were meant to be straightforward. If you found that those examples of primary alkyl halides were too easy, then have a look at some of these trickier examples below. (Not all of these will necessarily be SN2! )
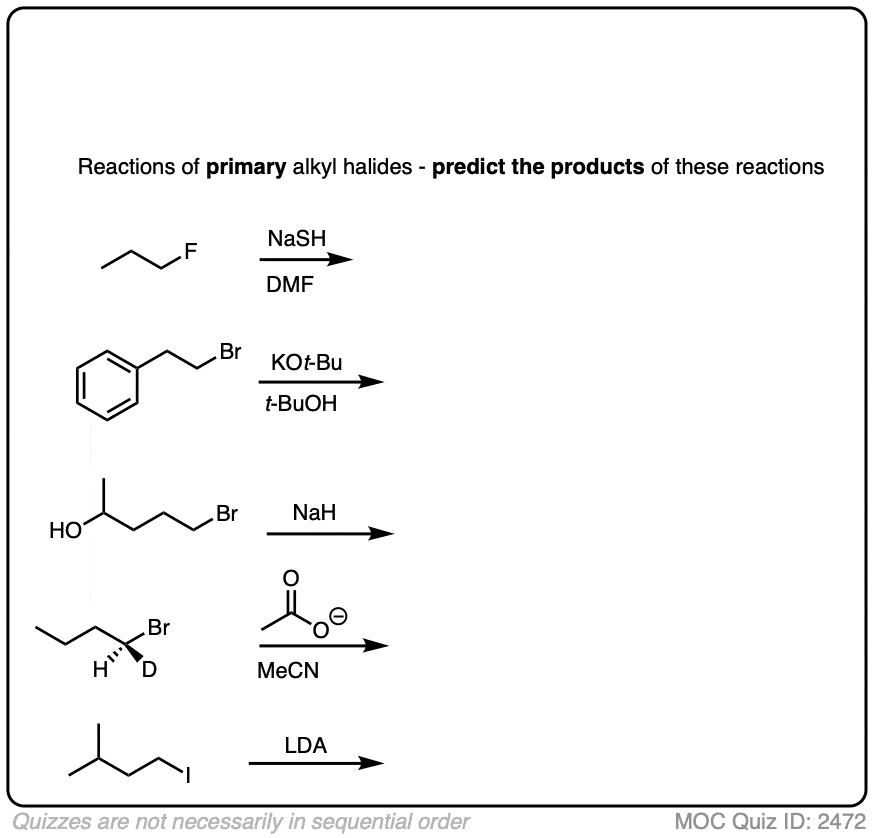 Click to Flip
Click to Flip
Some additional things to note here:
- Alkyl fluorides do not generally undergo substitution or elimination reactions under normal conditions due to the strong C-F bond.
- Sodium hydride (NaH) is a strong base but a poor nucleophile. It doesn’t attack the carbon to displace a leaving group, but it will deprotonate alcohols to give alkoxides (RO(-) ) which are excellent nucleophiles. [Never forget – the conjugate base is always a better nucleophile! ]
- There is only one situation where another reaction can compete with the SN2 on a primary alkyl halide, and that is when a strong, bulky base such as the t-butoxide ion (e.g. KOt-Bu) or lithium diisopropyl amide (LDA) is used. In these cases, the SN2 is slower than normal due to the steric bulk of the nucleophile, and elimination (E2) may start to compete with SN2. [Note 2]
4. So The Alkyl Halide Is Tertiary – What Then?
Secondary alkyl halides are a bit thorny, so let’s skip them for a moment and move straight to tertiary.
If the substrate is tertiary, you can rule out SN2. Why?
SN2 reactions generally don’t happen on tertiary alkyl halides because the SN2 proceeds through a backside attack (to the C-LG sigma* orbital) , and the backside of tertiary alkyl halides is too sterically hindered for SN2 reactions to occur at any meaningful rate. (See post – Steric Hindrance is Like a Fat Goalie)
Since SN2 is out of the picture, we’re left trying to choose between SN1, E1, and E2.
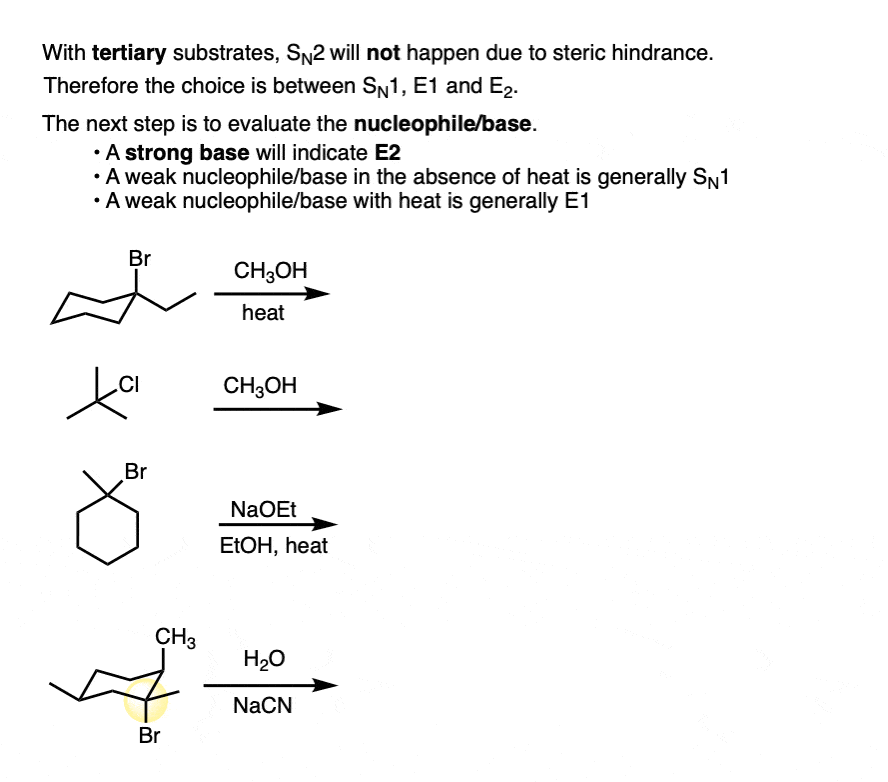
So what then?
I will cover this in the following article, but I don’t think I’m spoiling anything by saying that the next step is to look for the presence of a good base. [See next article – Deciding SN1/SN2/E1/E2 – The Nucleophile /Base]
By “good base”, I generally mean charged species at least as basic as alkoxides (RO– ) or greater. [although I would also include tertiary amines Note 3 ]
Seeing “heat” or the delta symbol ” Δ” is also a good clue that elimination is going on. [See article – Elimination Reactions Are Favored By Heat]
In the absence of a strong base, expect SN1/E1 with tertiary alkyl halides.
That just opens up another question, of course. How do you tell the difference between SN1 or E1?
This will also be covered in another subsequent article (See article – Deciding SN1/SN2/E1.E2 – The Role of Temperature), but the short answer is, “heat“. If heat is applied, then expect the reaction to be E1.
In the quiz below, which is collected from the examples above, I’ve actually done the work of identifying the most likely reaction pathway. Your job is to just draw the products.
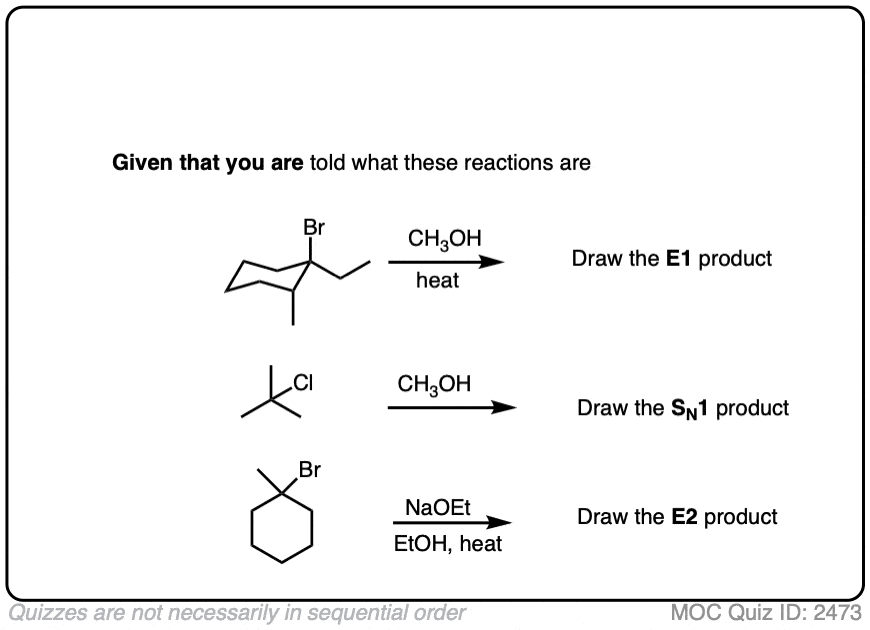 Click to Flip
Click to Flip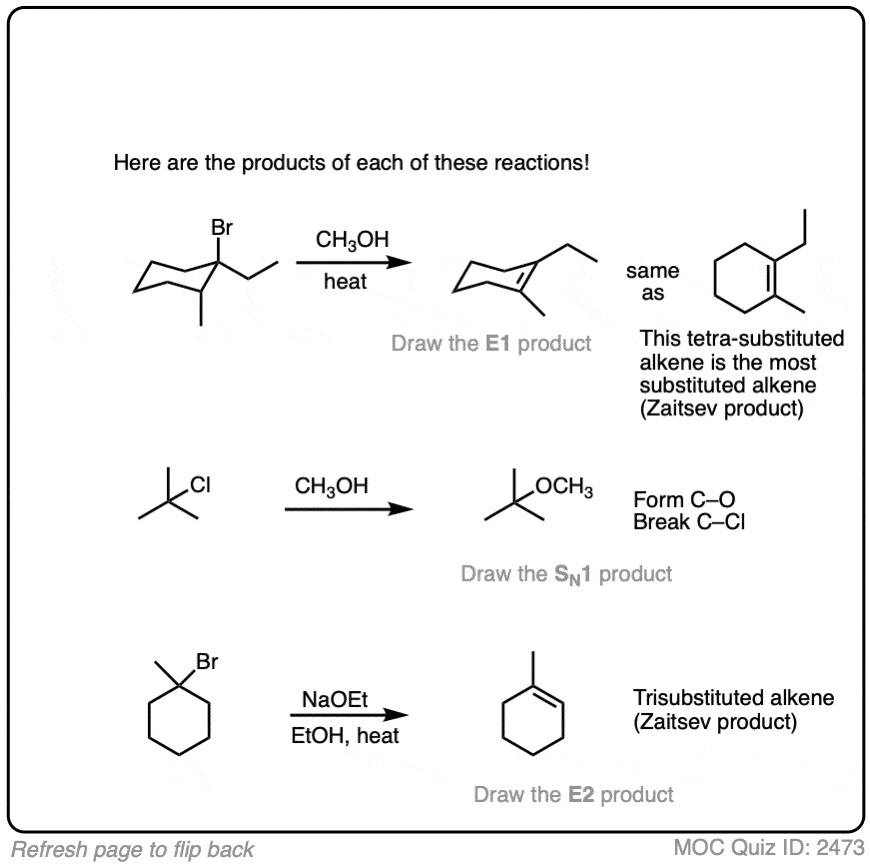
See this Note 4 for the slightly more difficult example.
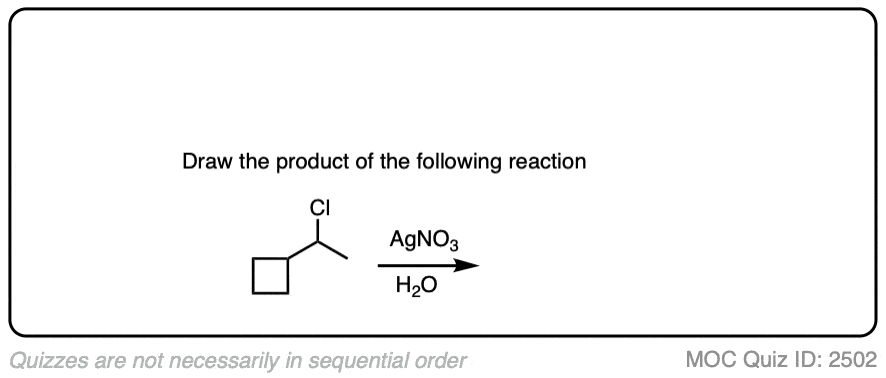
5. It’s Secondary – Now What?
Finally we get to the “fun” part: secondary alkyl halides. (Note – your definition of “fun” may vary)
The short news is that for secondary alkyl halides, we cant rule anything out. Depending on conditions, each of the four reaction patterns could potentially be observed.
I’m not going to be able to summarize the whole range of possibilities in one paragraph, but the next key question will be to examine the identity of the base / nucleophile.
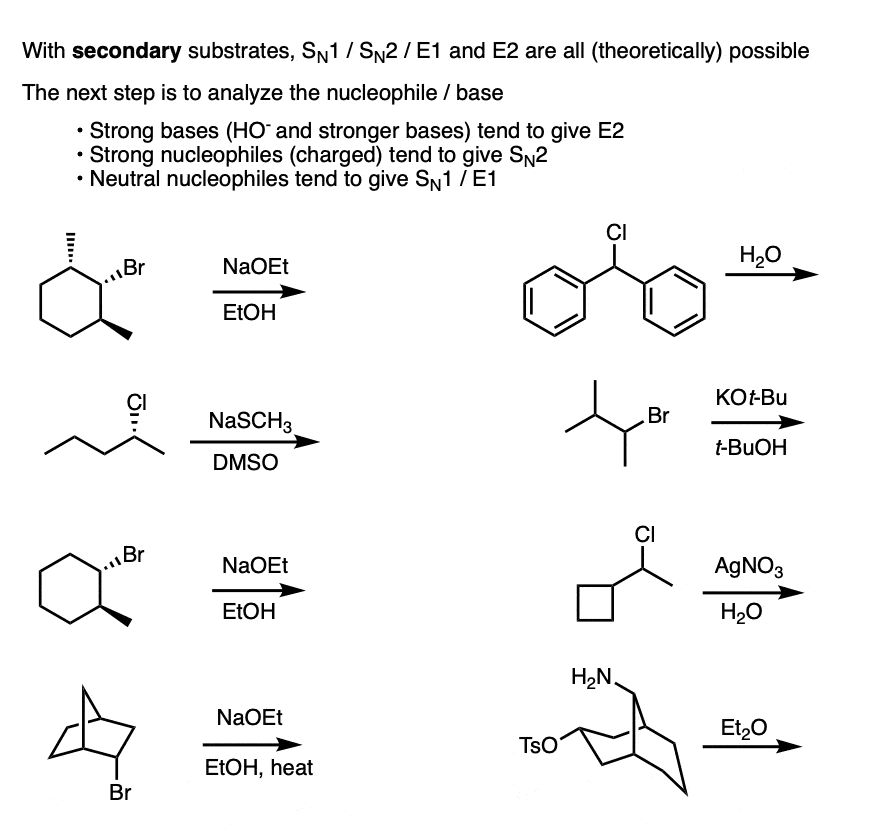
- Generally speaking, if the nucleophile/base is “strong” (and for our purposes, this means, “has a negative charge”) then expect SN2 or E2
- If the nucleophile/base is “weak” (neutral, for our purposes) then expect SN1 / E1.
Distinguishing SN2 vs E2 on a secondary alkyl halide is worth a whole other article on its own.
For now, let’s just note that the E2 requires a strong base, whereas the SN2 requires a strong nucleophile.
By “strong” I would suggest hydroxide ion (HO – , the conjugate base of water, pKa = 14) as a good cutoff point.
- Thiolates, the conjugate bases of thiols (pKa 10) tend to perform substitution reactions on secondary alkyl halides, as do less basic species such as N3(-), CN(-), halides, and carboxylates. (For a good list of nucleophiles, see post – Why The SN2 Is Powerful)
- Ssecondary alkyl halides treated with alkoxides are a particularly tricky case, as this is the borderline region between SN2 and E2. For a good time, ask your instructor on what they think will happen. (My personal observation is that polar aprotic solvents such as DMSO, DMF, and acetonitrile indicate SN2 whereas polar protic solvents indicate E2, but there are exceptions).
- Nucleophiles that are more basic than alkoxides tend to give E2 with secondary alkyl halides. Acetylides, the conjugate bases of terminal alkynes are a classic example here. [Note 5]
Here are some of the simpler examples from above. The more complex ones I’ll cover in the notes below.
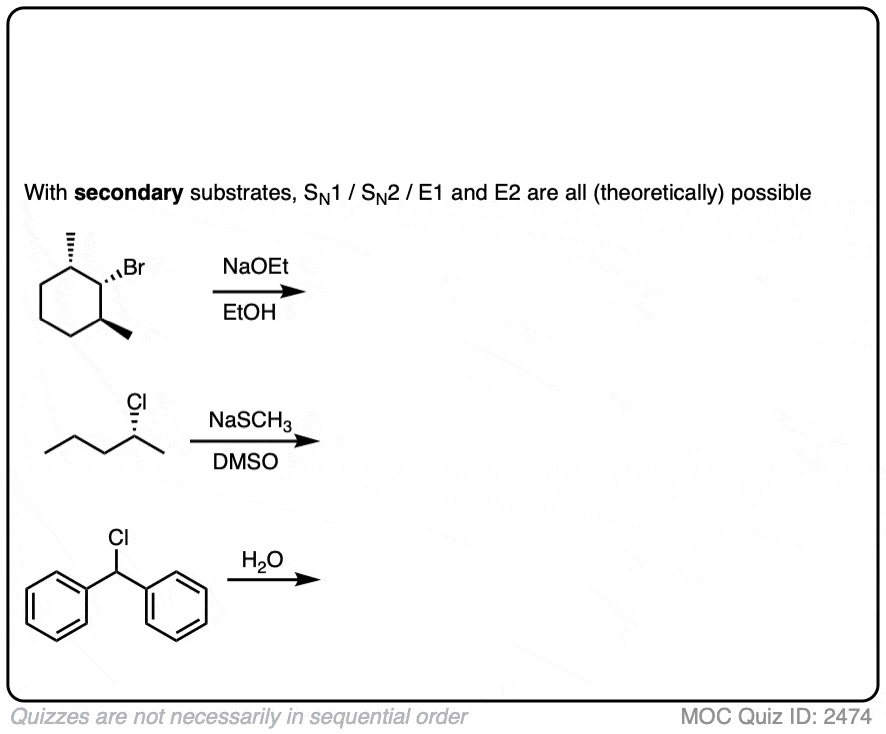 Click to Flip
Click to Flip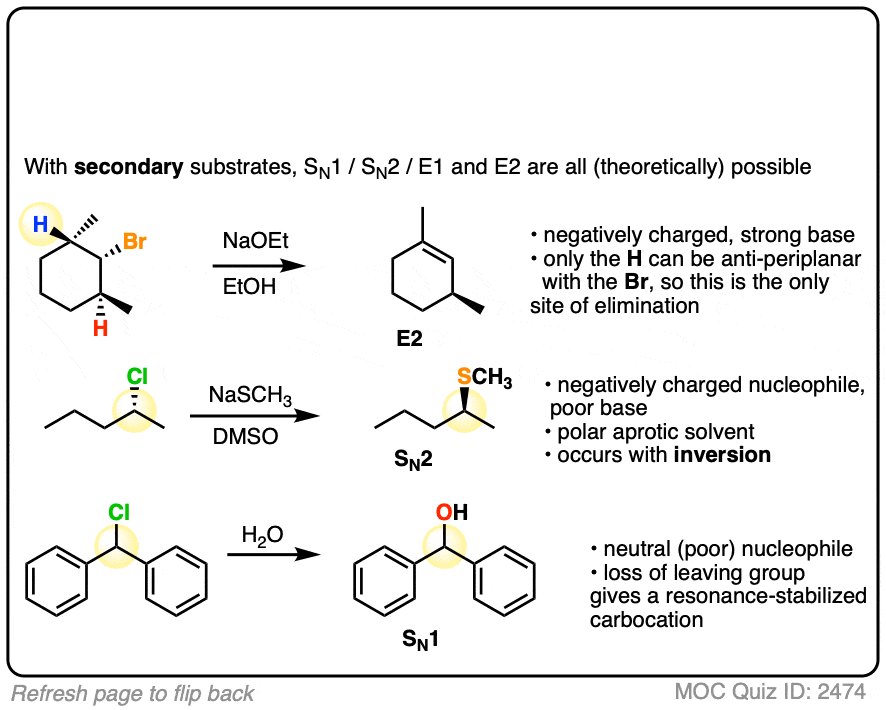
I’ll handle some of the more complicated cases of secondary alkyl halides in the notes below.
6. A Look Ahead
Although this is intended to be just one article in a sequence that walk through the process of deciding SN1/SN2/E1/E2, I appreciate that not everyone will go through this sequentially.
This is an outline of the thought process:
- The role of the substrate (this article)
- The role of the nucleophile/base (next! )
- The role of the solvent (SN2 vs E2 with secondary alkyl halides)
- The role of temperature (SN1 vs E1)

7. Some Examples With Alcohols
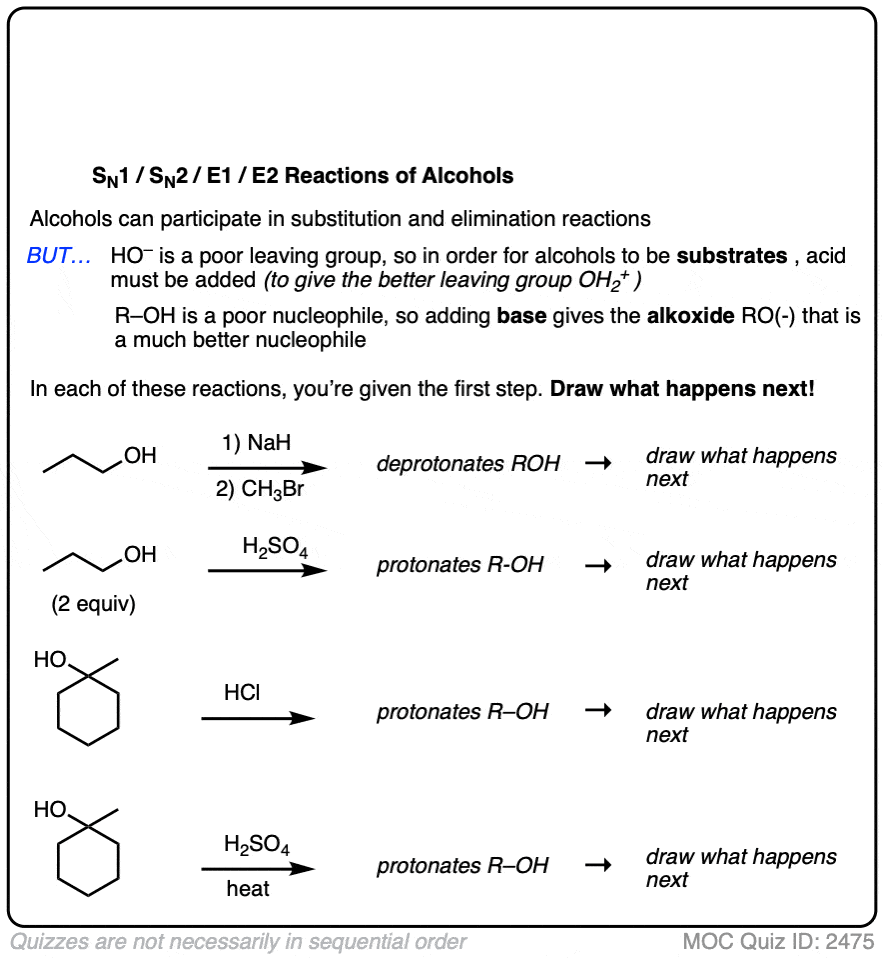
We’ve been talking about reactions of alkyl halides, but these reactions can happen with alcohols as well.
In their neutral form alcohols are poor nucleophiles and are also poor substrates for SN1/SN2/E1/E2 reactions since the hydroxide group (HO–) is a poor leaving group.
However, adding an acid or a base can change the alcohol’s personality dramatically!
- Treating an alcohol with a base makes the alcohol into an alkoxide, which is a far better nucleophile than a neutral alcohol.
- Treating an alcohol with an acid protonates the hydroxyl group R–OH into R–OH2(+) which is a far better leaving group than HO(-). This means that protonated alcohols can be electrophiles in SN1/SN2/E1 reactions (but generally not E2, since strong acids aren’t compatible with strong bases!)
 Click to Flip
Click to Flip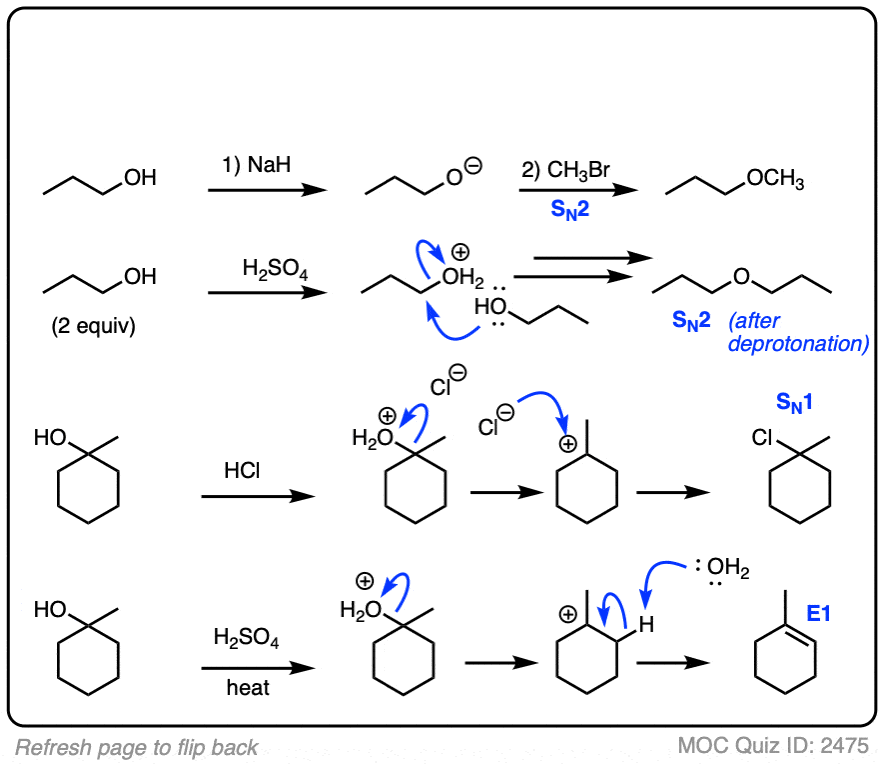
More on the substitution and elimination reactions of alcohols in the alcohol chapter. (Start here, for example)
8. Summary
- Identifying the type of substrate (primary, secondary, tertiary or methyl halide) is the first step towards identifying a reaction as SN1/SN2/E1/E2.
- I personally find it more helpful to look at it from the perspective of ruling things out rather than the inverse.
- If the alkyl halide is primary, you can rule out SN1 and E1, and rule out E2 unless the nucleophile is a very bulky base.
- If the alkyl halide is tertiary, you can rule out SN2.
With secondary alkyl halides, you can’t rule out any pathway in some cases without looking at the nucleophile/base.
That’s next!
Notes
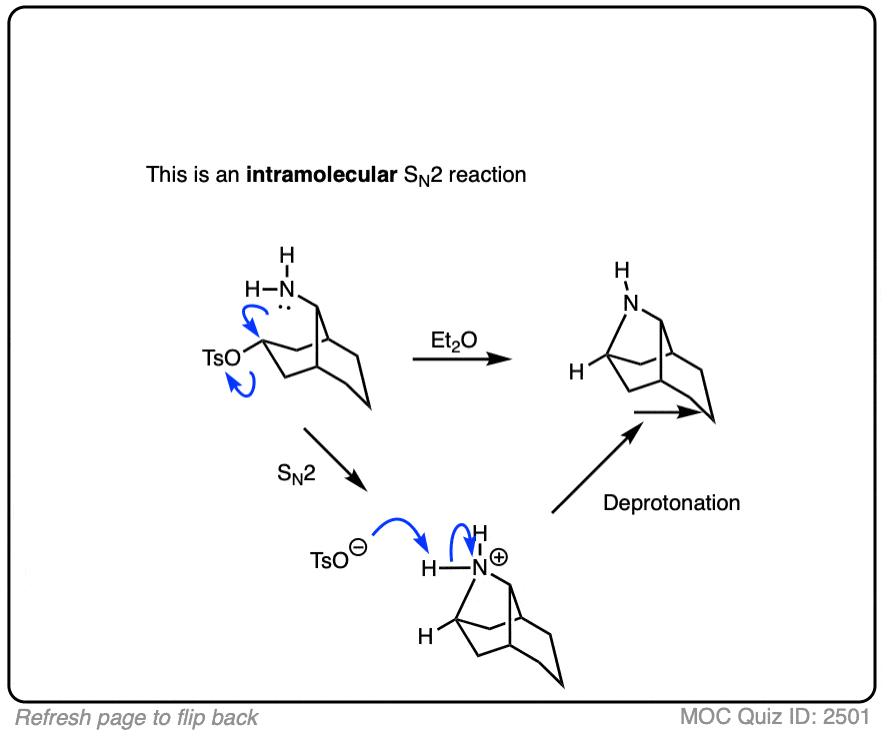
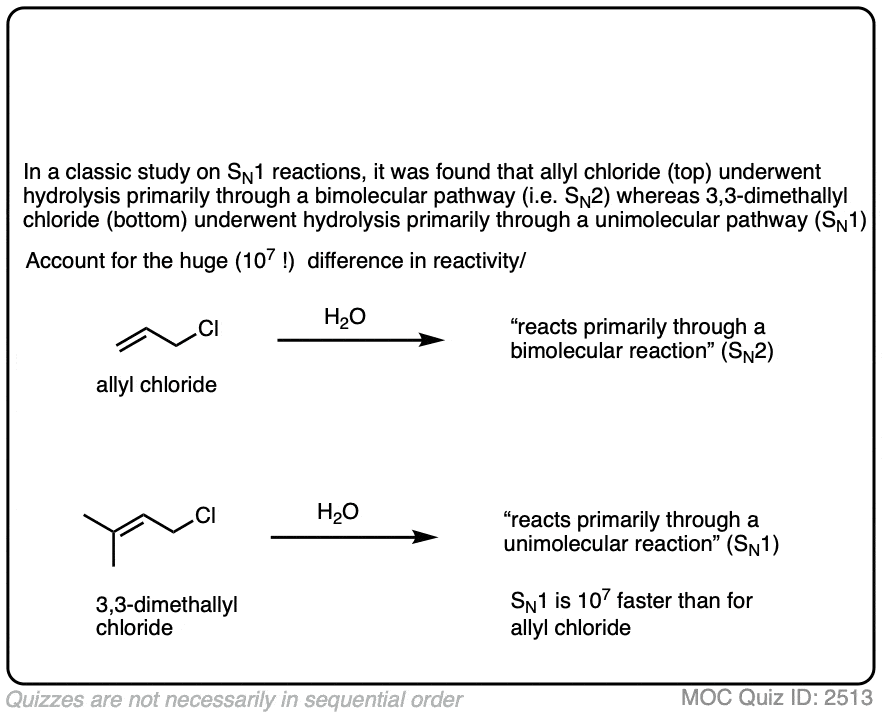
Note 1. It is also possible for the SN1 pathway to operate with primary allylic halides that are capable of forming extremely stable carbocations, particularly in very polar solvents such as mixtures of water and ethanol or formic acid.
One classic example is gamma, gamma dimethylallyl chloride, where the SN1 pathway is dominant.
 Click to Flip
Click to Flip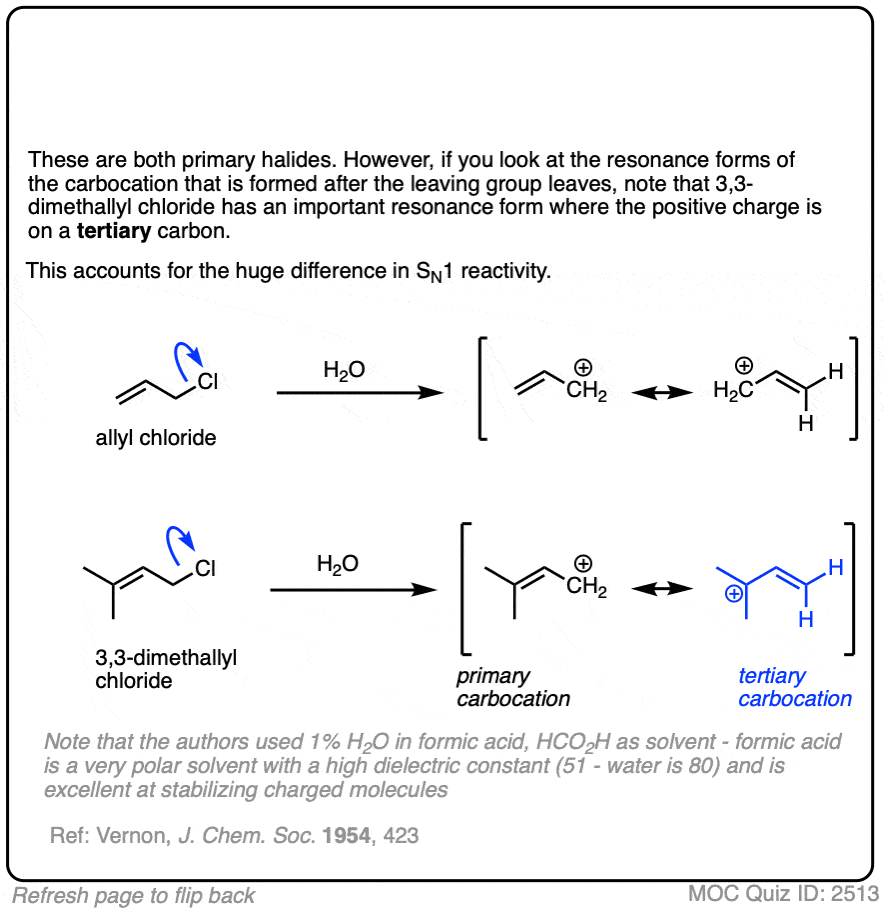
Note 2. Note that I’m not saying here that E2 will be the exclusive (100%) product, just that the percentage of E2 product will start to increase relative to SN2. Mixtures of products are fairly common in organic chemistry.
Note 3. Tertiary amines (R3N) are one example of an exception to this rule of thumb, as they are neutral species yet competent bases.
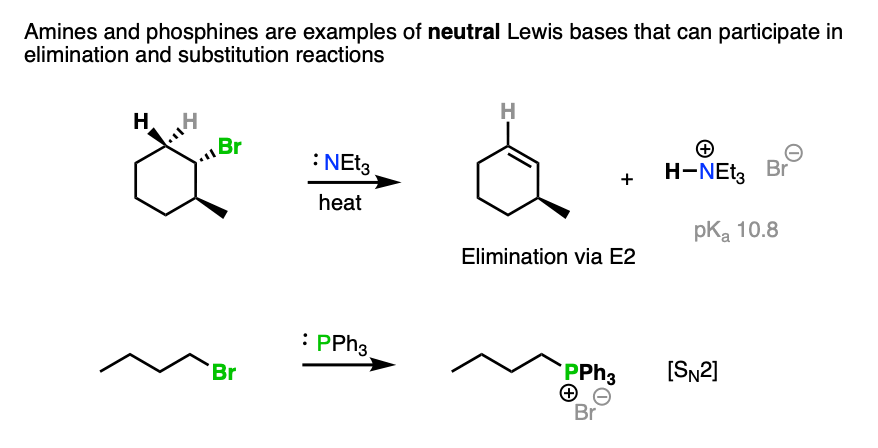
Phosphines (e.g. triphenylphosphine, PPh3) are poor bases but are excellent nucleophiles in SN2 reactions.
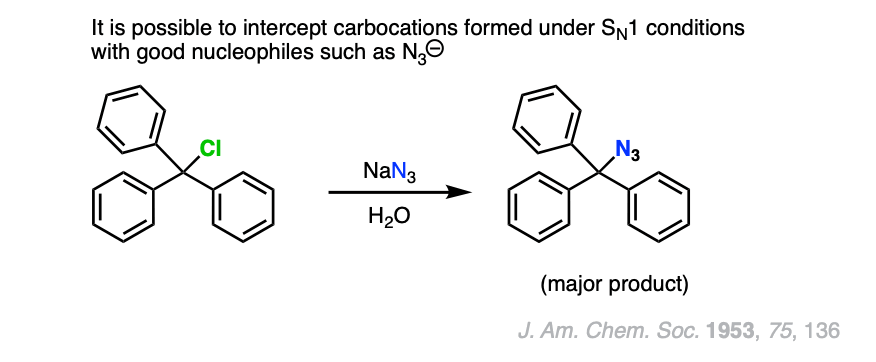
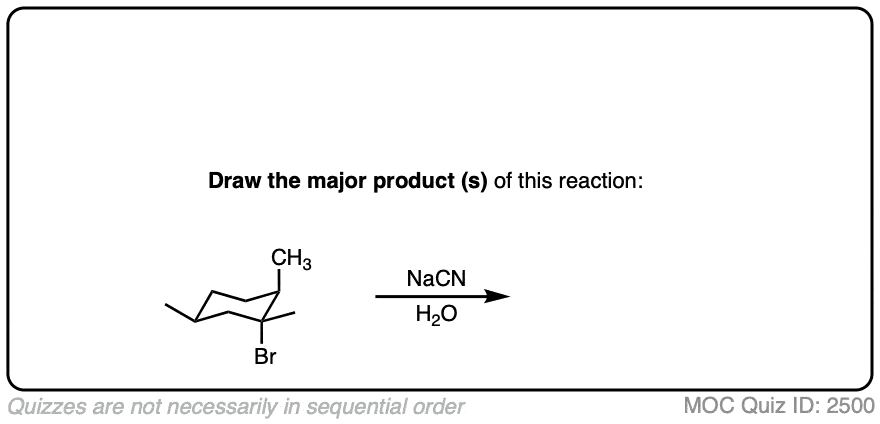
Note 4. If a carbocation is formed in the presence of a species that is a good nucleophile but a poor base, such as cyanide or azide ion, then these good nucleophiles can “intercept” the carbocation and react with it in place of the solvent.
Note 5. When secondary alkyl halides are treated with acetylides, the major product tends to be elimination rather than substitution products.
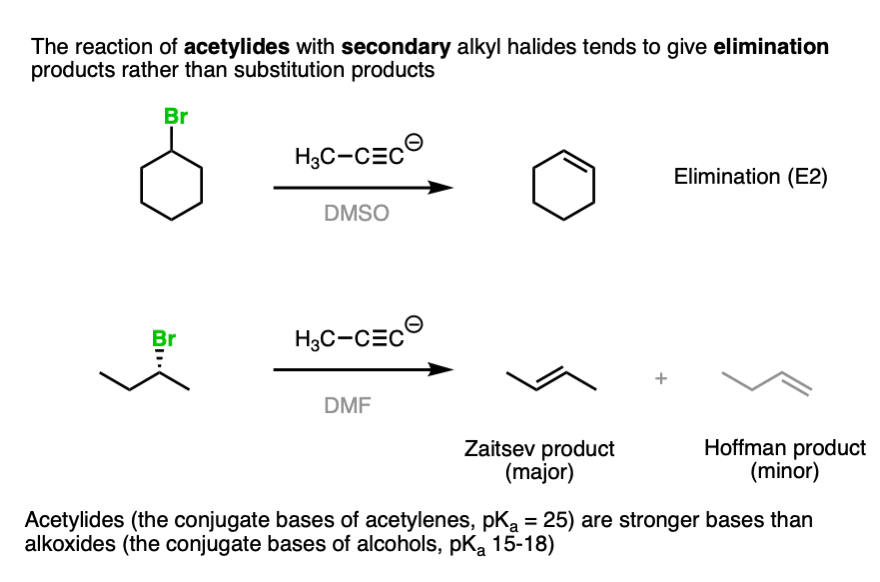
For examples, see this reference.
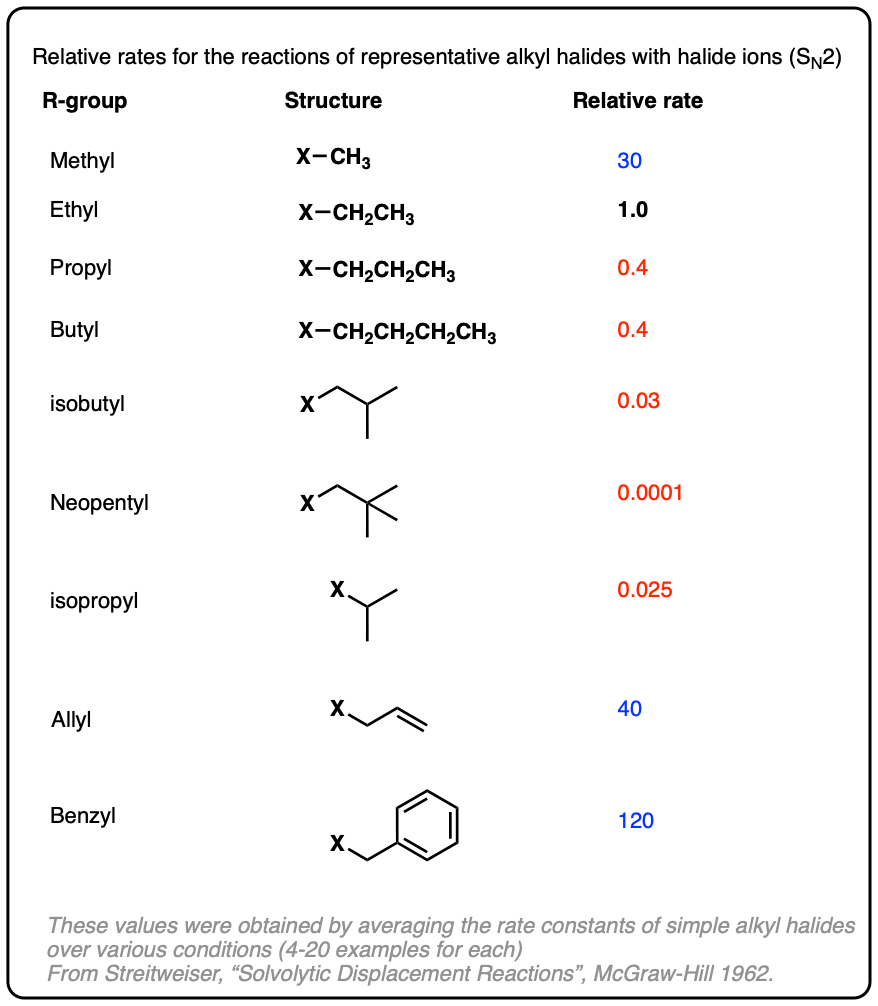
Note 6. This is a handy table of relative rates for various alkyl halides in substitution reactions with halide ions.
Quiz Yourself!
 Click to Flip
Click to Flip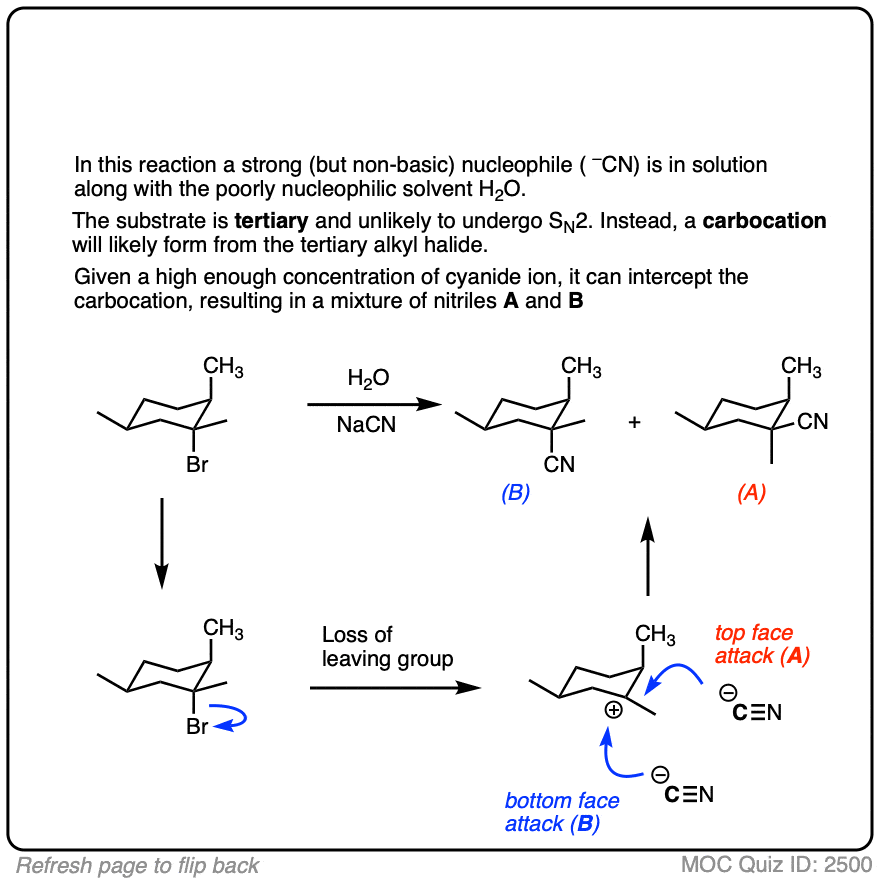
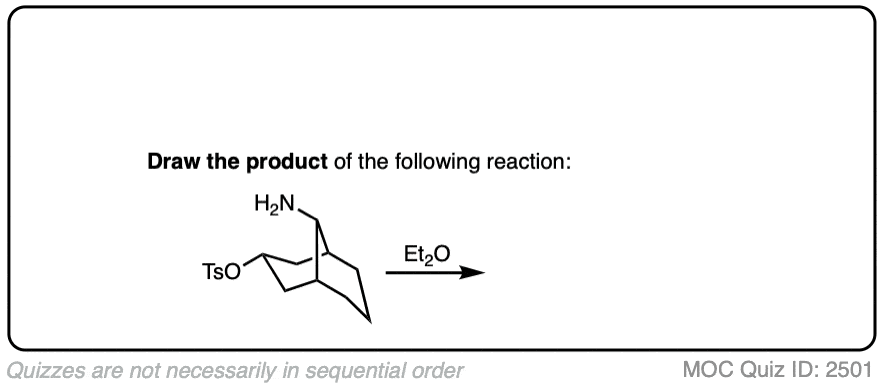 Click to Flip
Click to Flip