In the last post we saw that cyclopropane and cyclobutane have an unusually high “ring strain” of 27 kcal/mol and 26 kcal/mol respectively. We determined this by comparing heats of combustion from rings of various sizes, and saw that the ΔHcombustion per CH2 was essentially constant as ring sizes went above 12.
Based on these calcuations, we saw that cyclopropane and cyclobutane are much more unstable than we might naively expect for an “unstrained” ring of that size. In addition, the C-C bond strength in cyclopropane is considerably weaker (65 kcal/mol) than we observe for a typical C-C bond (80-85 kcal/mol).
Therefore, there must be some structural feature of cyclopropane and cyclobutane which leads to this additional strain.
What might that be? Let’s take a look.
Table of Contents
- Angle Strain In Cyclopropane and Cyclobutane
- Torsional Strain In Cyclopropane
- Torsional Strain In Cyclobutane – Some Puckering Is Possible
- Summary: Ring Strain In Cyclopropane and Cyclobutane
- Notes
- (Advanced) References and Further Reading
1. Angle Strain In Cyclopropane And Cyclobutane
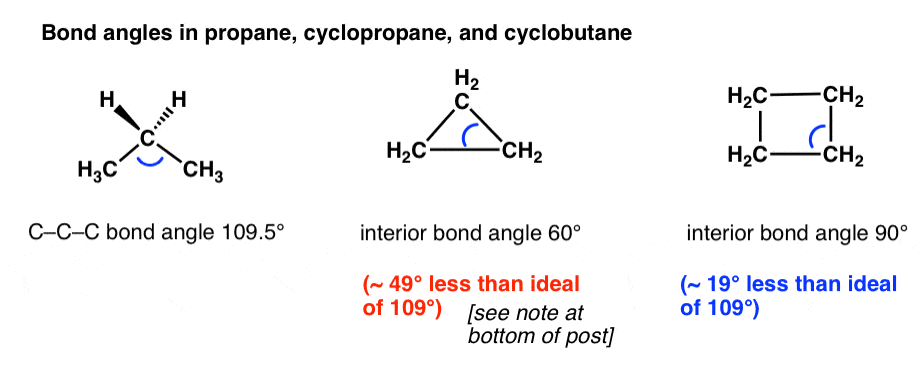
The first thing to notice about cyclopropane and cyclobutane is the non-ideal bond angles. The ideal bond angle in tetrahedral carbon is 109 degrees (See post: How Do We Know Methane Is Tetrahedral)
However, constrained to a triangle and a square, the interior angles of cyclopropane (60°) and cyclobutane (90°) are considerably less than the ideal angle.
This means that the electron clouds surrounding each atom will be considerably closer together than ideal, and since like charges repel, this will be energetically less favorable than for a straight-chain alkane.
The additional instability caused by this constraint is called angle strain or Von Baeyer strain.
[ Note – although the atoms of cyclopropane do form a triangle, the electron clouds between each atom do not necessarily follow the “lines” of it. The nature of the bonding in cyclopropane is a deep topic – Mike Evans put together a video on it here.]
So is it that simple? Is angle strain all there is to talk about? Not quite – there’s one more factor to consider.
2. Torsional Strain In Cyclopropane
From earlier chapters on conformations, you may recall the concept of “Torsional Strain” (aka “twisting strain) (See Article: Staggered vs. Eclipsed Conformations of Ethane).
Although those two words often generate confusion in students’ minds, the concept is very simple: strain generated through simple rotation.
Have you ever flown a toy plane with a wind-up propeller? As you twist the propeller, you will progressively encounter more and more resistance from the elastic bands.
When wind-up is complete, you can really feel that propeller digging into your finger! The elastic bands in this case are under a lot of torsional strain. Releasing the propeller results in untwisting of the elastic bands, which is the driving force for propelling the plane.
It is much the same with molecules.
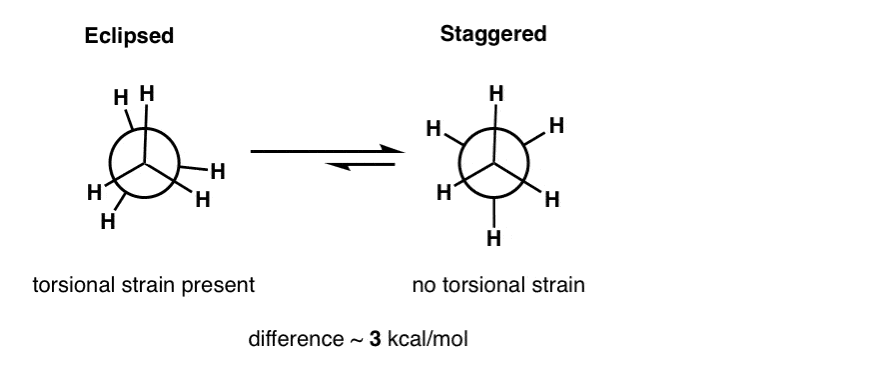
In ethane, for example, rotation about the C-C single bond results in two major conformations: the “eclipsed” conformation, where the two CH3 groups are directly aligned with each other along the C-C axis, and the more favorable “staggered” conformation where they are offset by 60 degrees.
The difference in energy between these two forms is about 3 kcal/mol (actually 2.8 kcal/mol).
Therefore ethane in the eclipsed conformation feels a torsional strain (driving force for rotation) of about 3 kcal/mol.
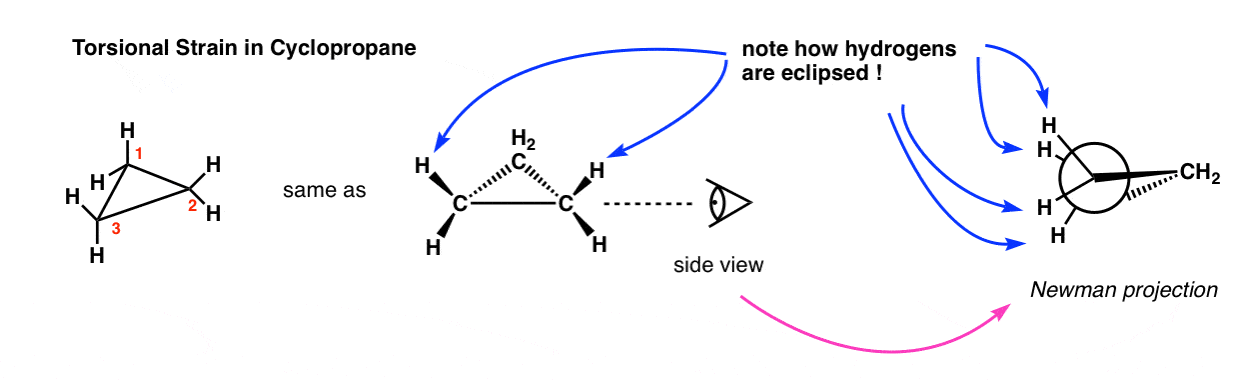
In cyclopropane, the adjcent CH2 groups are also eclipsed. Unlike in ethane, this strain cannot be relieved through rotation (the ring is too rigid).
In other words, the CH2 groups are locked in the eclipsed conformation, which results in torsional strain – much like a propeller that has been wound up but held in position. Much like wound-up propellers, it’s common to think of cyclopropanes as being “spring loaded” – later on we will see some examples of reactions where release of ring strain is a significant driving force!
3. Torsional Strain In Cyclobutane: Some Puckering Is Possible
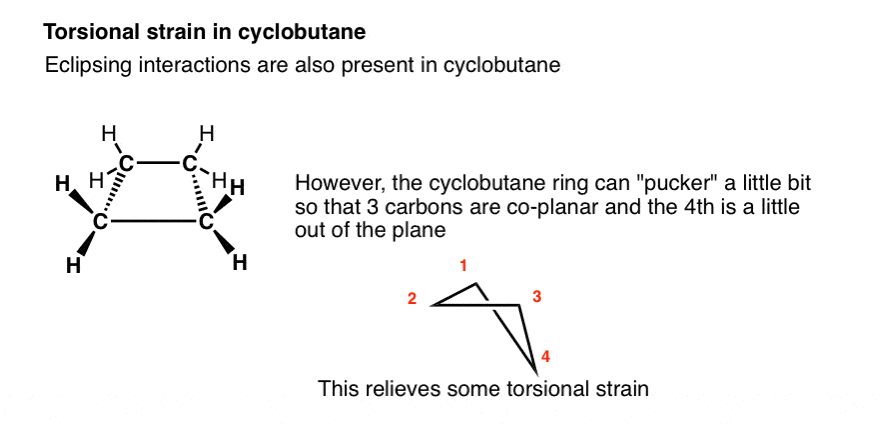
What about cyclobutane? If cyclobutane were completely flat, we would expect eclipsing interactions between four CH2 groups.
In reality, cyclobutane has a little bit of wiggle room. The result is that there are three carbons in one plane with the fourth appearing like a “flap” slightly out of plane.
Any one of the four carbon atoms can be the “flap” – in solution, there is interconversion between different conformers, and on average, each carbon spends an equal amount of time as the “flap”. The fact that cyclobutane rings can “pucker” like this leads to a small reduction in torsional strain.
4. Summary: Ring Strain In Cyclopropane and Cyclobutane
Both cyclopropane and cyclobutane have large ring strain due to a mixture of angle strain and torsional strain.
Be on the lookout for future reactions that have “relief of ring strain” as a driving force.
In the next post we’ll talk about 5 and 6 membered rings.
Here’s a puzzle for you: the interior angles of a pentagon are 108° whereas those of a hexagon are 120°.
Based on that alone, you might expect cyclopentane to be less strained than cyclohexane, since the angle is closer to the ideal angle of tetrahedral carbon.
In fact the exact opposite is the case. Why? Answer in the next post.
Next post: Ring Strain of Cyclopentane and Cyclohexane
