Alkyl Shifts In Carbocation Rearrangement Reactions, Including Ring Expansion
- Hydride shifts can sometimes occur when a more stable carbocation can be formed through migration of a C-H bond.
- If no hydride shift is possible that will result in a more stable carbocation, then it is possible that an alkyl shift can occur, where a C-C bond breaks and a C-C bond breaks. The net effect is the shifting over of the carbocation to the adjacent carbon.
- Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation
- Alkyl shifts adjacent to strained rings (e.g. cyclobutane) can result in ring expansion
Table of Contents
- Alkyl Shifts Can Lead To More Stable Carbocations, Too
- The Mechanism Of Alkyl Shift Reactions
- Example of An SN1 With Alkyl Shift
- Ring-Expansion Reactions Also Involve Alkyl Shifts
- Notes
1. Alkyl Shifts Can Lead To More Stable Carbocations, Too
In the previous post we saw how certain carbocations can sometimes rearrange (through hydride shifts) to give more stable carbocations (See post: Hydride Shifts)
However, sometimes there are situations where a hydride shift would not lead to a more stable carbocation, but an alkyl shift would!
Take a look at this carbocation. If a hydride shift occurred here, we’d be going to a less stable (primary) carbocation! Not going to happen.
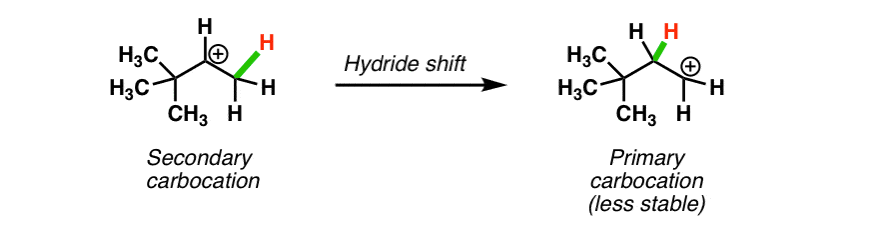
You might note something with this example, however: it is possible for a more stable tertiary carbocation to be formed.
How? If an alkyl group migrates instead! (Look at that red methyl group! )
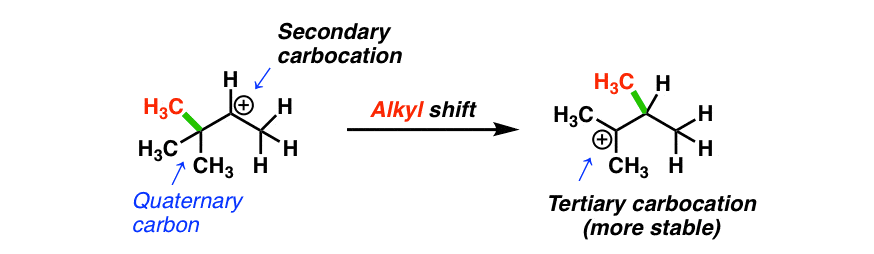
The most common situation where alkyl shifts can occur is when a quaternary carbon (that’s a carbon attached to 4 carbons) is adjacent to a secondary carbocation. (See post: Primary, Secondary, Tertiary, Quaternary)
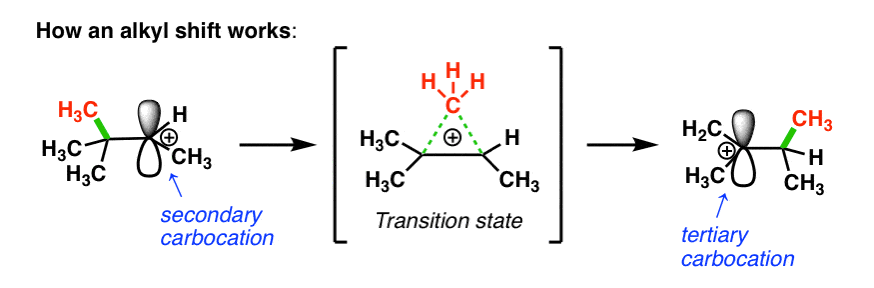
2. The Mechanism Of Alkyl Shift Reactions In Carbocation Rearrangements
How does this work? First, the pair of electrons from the C-C bond must align with the empty p orbital on the carbocation (side note: this means they have to be aligned in the same plane in order for orbital overlap to occur).
Then, as the pair of electrons from the C–C bond is donated into the empty p-orbital, one C–C bond begins to break and the new C–C bond begins to form.
In the transition state, there are partial bonds between the carbon being transferred and each of the two adjacent carbon atoms. Then, as one bond shortens and the other lengthens, we end up with a (more stable) tertiary carbocation. (See post: 3 Factors That Stabilize Carbocations)
Rearrangements can potentially occur any time a carbocation is formed. That includes SN1 reactions (and as we’ll later see, elimination and addition reactions).
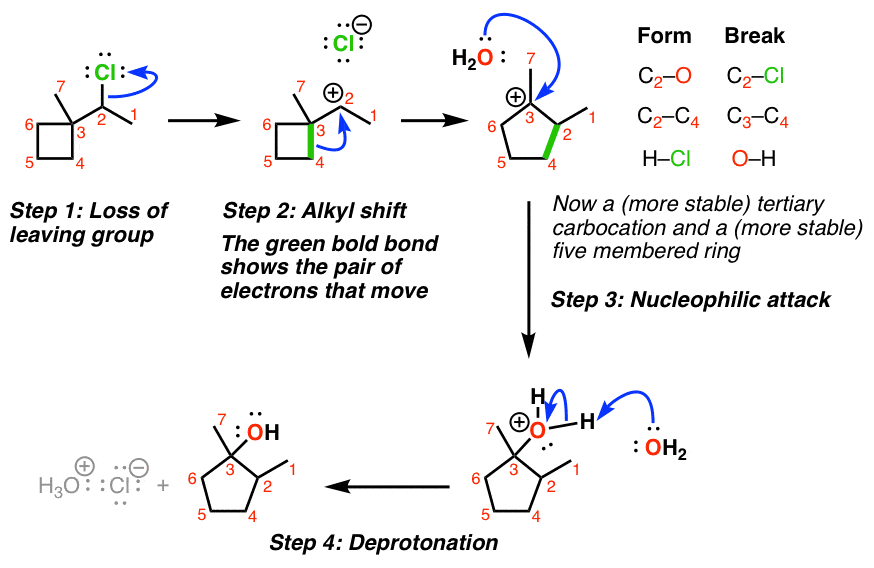
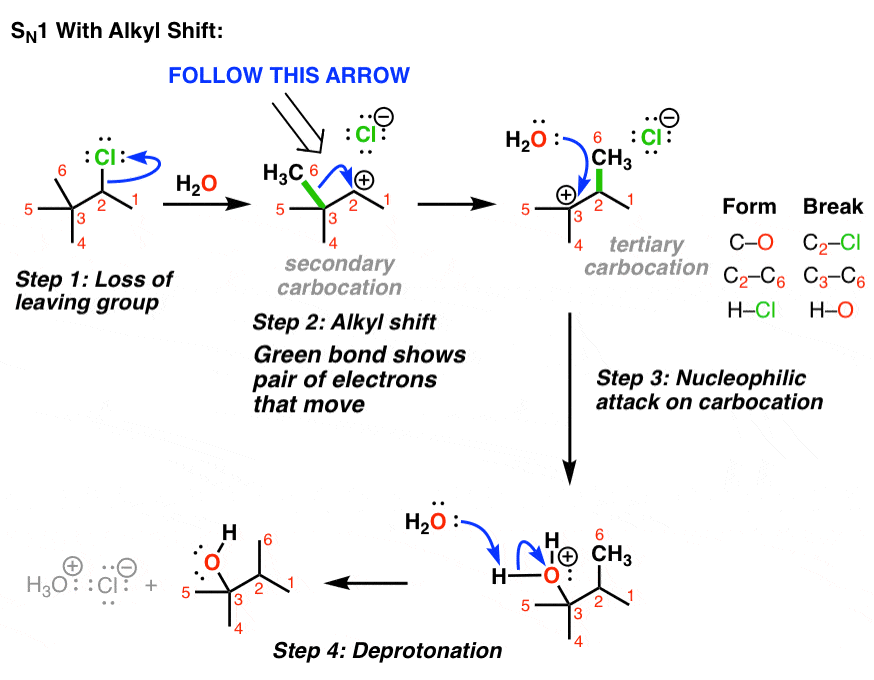
3. Example of An SN1 With Alkyl Shift
4. Ring-Expansion Reactions Also Involve Migration of Carbon
It doesn’t always have to be a methyl group that moves! One interesting example is when a carbocation is formed adjacent to a strained ring, such as a cyclobutane. (See: Ring Strain in Cyclopropane and Cyclobutane)
Even though the CH3 could potentially migrate in this case, it’s more favorable to shift one of the alkyl groups in the ring, which leads to ring expansion and the formation of a less strained, five-membered ring.
Here’s an example of an SN1 where migration of a carbon-carbon bond leads to ring expansion.